Chemistry: Matter and Change
... What happens when an element emits radioactive particles? A. It gains energy. B. It gains neutrons. ...
... What happens when an element emits radioactive particles? A. It gains energy. B. It gains neutrons. ...
Chapter 2 – Atoms and Elements
... Elements in the same period have similar ___________ but very different ________________. Elements in the same group have similar _________________ but very different ____________. Despite having similar chemical properties, elements in the same group sometimes have quite different physical properti ...
... Elements in the same period have similar ___________ but very different ________________. Elements in the same group have similar _________________ but very different ____________. Despite having similar chemical properties, elements in the same group sometimes have quite different physical properti ...
Chapter 2 – Atoms and Elements
... Elements in the same period have similar ___________ but very different ________________. Elements in the same group have similar _________________ but very different ____________. Despite having similar chemical properties, elements in the same group sometimes have quite different physical properti ...
... Elements in the same period have similar ___________ but very different ________________. Elements in the same group have similar _________________ but very different ____________. Despite having similar chemical properties, elements in the same group sometimes have quite different physical properti ...
Unit 3 Chap. 3 Atoms: The Building Blocks of Matter
... A New System of Chemical Philosophy (1808) 1. Each element is made up of tiny particles called atoms. 2. The atoms of a given element are identical and different from atoms of other elements. 3. Atoms cannot be subdivided, created, or destroyed. 4. Chemical compounds are formed when atoms combine wi ...
... A New System of Chemical Philosophy (1808) 1. Each element is made up of tiny particles called atoms. 2. The atoms of a given element are identical and different from atoms of other elements. 3. Atoms cannot be subdivided, created, or destroyed. 4. Chemical compounds are formed when atoms combine wi ...
Chapter 5 Atomic Structure and the Periodic Table
... tube. A cathode ray tube is similar to your TV. It has an anode (negative electrode) and a cathode (positive electrode). These are enclosed in an evacuated (air removed) glass container and when a charge is applied the electrons flow from anode to cathode through the open space of the glass containe ...
... tube. A cathode ray tube is similar to your TV. It has an anode (negative electrode) and a cathode (positive electrode). These are enclosed in an evacuated (air removed) glass container and when a charge is applied the electrons flow from anode to cathode through the open space of the glass containe ...
NOTES Atomic Structure Number Mass.docx
... measure – atomic mass. Atomic mass is the relative average mass of an atom of the element. There are no mass units for atomic mass. They are simply a ratio. Carbon has an atomic mass of 12, so it is 12 times heavier than hydrogen, which is 1. Oxygen atoms have 16 times more mass than hydrogen. John ...
... measure – atomic mass. Atomic mass is the relative average mass of an atom of the element. There are no mass units for atomic mass. They are simply a ratio. Carbon has an atomic mass of 12, so it is 12 times heavier than hydrogen, which is 1. Oxygen atoms have 16 times more mass than hydrogen. John ...
PS 2.3
... For example, all elements in period 4 have four occupied energy levels. This is an introduction to quantum theory that will be studied in chemistry. Recognize a given element’s atomic mass (the weighted average of the masses of the naturally occurring isotopes of the element), by recognizing tha ...
... For example, all elements in period 4 have four occupied energy levels. This is an introduction to quantum theory that will be studied in chemistry. Recognize a given element’s atomic mass (the weighted average of the masses of the naturally occurring isotopes of the element), by recognizing tha ...
Chapter 4 – Atoms
... Masses of atoms are so small that we define the atomic mass unit (amu) to scale up the numbers. Carbon-12 was chosen as the reference and given a mass value of exactly 12.000 amu. The mass of all other atoms are scaled relative to mass of Carbon-12. The Atomic Mass of an Element in the Periodic Tabl ...
... Masses of atoms are so small that we define the atomic mass unit (amu) to scale up the numbers. Carbon-12 was chosen as the reference and given a mass value of exactly 12.000 amu. The mass of all other atoms are scaled relative to mass of Carbon-12. The Atomic Mass of an Element in the Periodic Tabl ...
Atomic mass
... different atomic masses. B. Atoms of the same element having different numbers of neutrons. Hydrogen has three isotopes: Protium – 0 neutrons Deuterium – 1 neutron Tritium – 2 neutrons ...
... different atomic masses. B. Atoms of the same element having different numbers of neutrons. Hydrogen has three isotopes: Protium – 0 neutrons Deuterium – 1 neutron Tritium – 2 neutrons ...
Chapter 4: The Structure of the Atom
... Does not consist of particles Gamma radiation accompanies alpha and beta radiation Much more penetrating than either of a ...
... Does not consist of particles Gamma radiation accompanies alpha and beta radiation Much more penetrating than either of a ...
EXPERIMENT 4 – The Periodic Table
... Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements are composed of atoms, the smallest units that are characteristic of a particular element. Some elements occur in different forms, such as graphite and diamon ...
... Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements are composed of atoms, the smallest units that are characteristic of a particular element. Some elements occur in different forms, such as graphite and diamon ...
EXPERIMENT 4 – The Periodic Table
... Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements are composed of atoms, the smallest units that are characteristic of a particular element. Some elements occur in different forms, such as graphite and diamon ...
... Primary substances, called elements, build all the materials around you. There are more than 109 different elements known today. The elements are composed of atoms, the smallest units that are characteristic of a particular element. Some elements occur in different forms, such as graphite and diamon ...
Atomic Number
... Protons and neutrons are responsible for most of the atomic mass of an atom, while electrons contribute a very small amount of mass(9.108 X 10-28 grams). ...
... Protons and neutrons are responsible for most of the atomic mass of an atom, while electrons contribute a very small amount of mass(9.108 X 10-28 grams). ...
Atomic masses are weighted averages.
... What we know now of Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms. Atoms are not indivisible – they are made of subatomic particles 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element ...
... What we know now of Dalton’s Atomic Theory 1. All elements are composed of tiny indivisible particles called atoms. Atoms are not indivisible – they are made of subatomic particles 2. Atoms of the same element are identical. The atoms of any one element are different from those of any other element ...
Atoms - Chemistry Land
... cold beaker above it, you can see water condensing on it. So the dry paper actually has water in it. Finally as it burns out you see the ashes, which is like dirt or earth. ...
... cold beaker above it, you can see water condensing on it. So the dry paper actually has water in it. Finally as it burns out you see the ashes, which is like dirt or earth. ...
only that they did. democritus, an early greek philosopher, even had
... Being asked what animal you'd like to be is a trick question; you're already an animal. Doug Coupland ...
... Being asked what animal you'd like to be is a trick question; you're already an animal. Doug Coupland ...
Example of calculating average atomic mass
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
... 2. Atoms of one element cannot be converted into atoms of another element in a chemical reaction. Elements can only be converted into other elements in nuclear reactions. 3. All atoms of an element have the same number of protons and electrons, which determines the chemical behavior of the element. ...
10_Chemistry homework
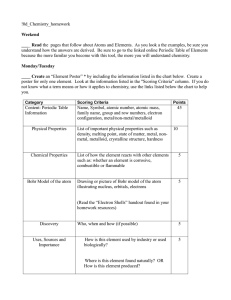
... ____ Create an “Element Poster” * by including the information listed in the chart below. Create a poster for only one element. Look at the information listed in the "Scoring Criteria" column. If you do not know what a term means or how it applies to chemistry, use the links listed below the chart t ...
... ____ Create an “Element Poster” * by including the information listed in the chart below. Create a poster for only one element. Look at the information listed in the "Scoring Criteria" column. If you do not know what a term means or how it applies to chemistry, use the links listed below the chart t ...
Atomic Structure and the Elements
... going across the table and the other is the Valence group number for 8 groups ...
... going across the table and the other is the Valence group number for 8 groups ...
1 | Page Chemistry Lecture #19: Atomic Number, Isotopes, and
... Protium has 1 proton in its nucleus. Deuterium has 1 proton and 1 neutron in its nucleus. Tritium has 1 proton and 2 neutrons in its nucleus. All 3 types of atoms have one proton in the nucleus. them are isotopes of hydrogen.. ...
... Protium has 1 proton in its nucleus. Deuterium has 1 proton and 1 neutron in its nucleus. Tritium has 1 proton and 2 neutrons in its nucleus. All 3 types of atoms have one proton in the nucleus. them are isotopes of hydrogen.. ...
U2notes2015
... same elements in the same proportions by mass even if we look at different samples 3. Dalton’s Law of multiple proportions: when 2 elements form a series of compounds, the ratios of the masses of nd the 2 element that combine with 1 gram of the first element can always be reduced to small whole numb ...
... same elements in the same proportions by mass even if we look at different samples 3. Dalton’s Law of multiple proportions: when 2 elements form a series of compounds, the ratios of the masses of nd the 2 element that combine with 1 gram of the first element can always be reduced to small whole numb ...
Nuclide, Atomic Number, mass number - Chemwiki
... Elements can also have isotopes with the same atomic number, but different numbers of neutrons. There may be a few more or a few less neutrons, and so the mass is increased or decreased. On the periodic table, the mass number is usually located below the element symbol. The mass number listed is th ...
... Elements can also have isotopes with the same atomic number, but different numbers of neutrons. There may be a few more or a few less neutrons, and so the mass is increased or decreased. On the periodic table, the mass number is usually located below the element symbol. The mass number listed is th ...
Chem-130 Test Lecture
... Atomic number is the number of protons in the nucleus. All atoms of the same element have the same atomic number. Mass number is the sum of the number of protons & neutrons The number of neutrons in the nucleus is given by the mass number minus the atomic number. Isotopes are atoms of the same ...
... Atomic number is the number of protons in the nucleus. All atoms of the same element have the same atomic number. Mass number is the sum of the number of protons & neutrons The number of neutrons in the nucleus is given by the mass number minus the atomic number. Isotopes are atoms of the same ...
Word List
... 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the periodic table. 1.12 I can write the names and symbols of selected transition metals, lanthanides and actinides (1B12B) on th ...
... 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the periodic table. 1.12 I can write the names and symbols of selected transition metals, lanthanides and actinides (1B12B) on th ...
Promethium

Promethium, originally prometheum, is a chemical element with symbol Pm and atomic number 61. All of its isotopes are radioactive; it is one of only two such elements that are followed in the periodic table by elements with stable forms, a distinction shared with technetium. Chemically, promethium is a lanthanide, which forms salts when combined with other elements. Promethium shows only one stable oxidation state of +3; however, a few +2 compounds may exist.In 1902, Bohuslav Brauner suggested there was an element with properties intermediate between those of the known elements neodymium (60) and samarium (62); this was confirmed in 1914 by Henry Moseley who, having measured the atomic numbers of all the elements then known, found there was an element with atomic number 61. In 1926, an Italian and an American group claimed to have isolated a sample of element 61; both ""discoveries"" were soon proven to be false. In 1938, during a nuclear experiment conducted at Ohio State University, a few radioactive nuclides were produced that certainly were not radioisotopes of neodymium or samarium, but there was a lack of chemical proof that element 61 was produced, and the discovery was not generally recognized. Promethium was first produced and characterized at Oak Ridge National Laboratory in 1945 by the separation and analysis of the fission products of uranium fuel irradiated in a graphite reactor. The discoverers proposed the name ""prometheum"" (the spelling was subsequently changed), derived from Prometheus, the Titan in Greek mythology who stole fire from Mount Olympus and brought it down to humans, to symbolize ""both the daring and the possible misuse of mankind's intellect"". However, a sample of the metal was made only in 1963.There are two possible sources for natural promethium: rare decays of natural europium-151 (producing promethium-147), and uranium (various isotopes). Practical applications exist only for chemical compounds of promethium-147, which are used in luminous paint, atomic batteries, and thickness measurement devices, even though promethium-145 is the most stable promethium isotope. Because natural promethium is exceedingly scarce, it is typically synthesized by bombarding uranium-235 (enriched uranium) with thermal neutrons to produce promethium-147.