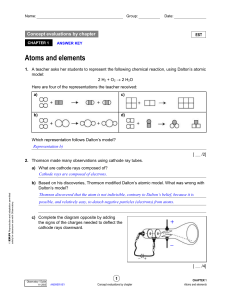
Homework #1 Atoms
... which have a positive charge; and _____________, which are neutral. The latter two particles are found in the ______________ of the atom. 2. It was __________________ who discovered the nucleus of the atom. The nucleus, which has a ______________ charge, occupies a very small volume of the atom. In ...
... which have a positive charge; and _____________, which are neutral. The latter two particles are found in the ______________ of the atom. 2. It was __________________ who discovered the nucleus of the atom. The nucleus, which has a ______________ charge, occupies a very small volume of the atom. In ...
Chemistry: Matter and Change
... surrounding an atom. Nuclear reactions involve changes in the nucleus of an atom. ...
... surrounding an atom. Nuclear reactions involve changes in the nucleus of an atom. ...
Atomic Structure – Study Guide
... 2. Neutrons -- a neutral charge (no charge) found in the nucleus. These determine whether an element is radioactive. Protons and neutrons are about one atomic mass unit (amu). Electrons have a much smaller mass -- it takes almost 2000 electrons to equal 1 amu. Atomic Mass = the total number of proto ...
... 2. Neutrons -- a neutral charge (no charge) found in the nucleus. These determine whether an element is radioactive. Protons and neutrons are about one atomic mass unit (amu). Electrons have a much smaller mass -- it takes almost 2000 electrons to equal 1 amu. Atomic Mass = the total number of proto ...
File
... Around 400 BCE, the Greek philosopher Democritus proposed that all matter can be divided into smaller and smaller pieces until a single indivisible particle is reached. He named this particle the atom (the smallest unit of an element). ...
... Around 400 BCE, the Greek philosopher Democritus proposed that all matter can be divided into smaller and smaller pieces until a single indivisible particle is reached. He named this particle the atom (the smallest unit of an element). ...
specific vocabulary of the unit
... Any of the elements of Group 18 (VIII, VIIIA), which includes helium, neon, argon, krypton, xenon, radon, and element 118. These elements are referred to as "inert" or "noble" because they do not easily form compounds with other elements. Transition elements /træn'zɪʃən//'eləmənts / A class of eleme ...
... Any of the elements of Group 18 (VIII, VIIIA), which includes helium, neon, argon, krypton, xenon, radon, and element 118. These elements are referred to as "inert" or "noble" because they do not easily form compounds with other elements. Transition elements /træn'zɪʃən//'eləmənts / A class of eleme ...
Chemistry: Matter and Change
... surrounding an atom. Nuclear reactions involve changes in the nucleus of an atom. ...
... surrounding an atom. Nuclear reactions involve changes in the nucleus of an atom. ...
Elements and Compounds
... the atmosphere AND differentiate between elements and compounds on the most basic level. ...
... the atmosphere AND differentiate between elements and compounds on the most basic level. ...
Using your periodic table ppt (9/26-10/11) File
... How to solve for ATOMIC MASS: • Atomic mass Scientists have established a standard for the measurement of atomic mass by assigning the carbon-12 atom a mass of 12 atomic mass units (amu). Thus, 1 amu is equal to 1/12 the mass of a carbon-12 atom. • The number at the bottom of each square in the per ...
... How to solve for ATOMIC MASS: • Atomic mass Scientists have established a standard for the measurement of atomic mass by assigning the carbon-12 atom a mass of 12 atomic mass units (amu). Thus, 1 amu is equal to 1/12 the mass of a carbon-12 atom. • The number at the bottom of each square in the per ...
11129_evl_ch1_ste_corr
... No, they do not all belong to the same period because they do not all have the same number of electron shells. Some of them (boron, nitrogen, fluorine and neon) have two electron shells; others (sodium and magnesium) have three. ...
... No, they do not all belong to the same period because they do not all have the same number of electron shells. Some of them (boron, nitrogen, fluorine and neon) have two electron shells; others (sodium and magnesium) have three. ...
atoms
... How then are atoms of one element different from another element? Elements are different because they contain different numbers of PROTONS The “atomic number” of an element is the number of protons in the nucleus # protons in an atom = # electrons in a neutral ...
... How then are atoms of one element different from another element? Elements are different because they contain different numbers of PROTONS The “atomic number” of an element is the number of protons in the nucleus # protons in an atom = # electrons in a neutral ...
What is an ion?
... Just as each state has a two-letter abbreviation, each element has a oneor two-letter symbol to make life simple for chemists. ...
... Just as each state has a two-letter abbreviation, each element has a oneor two-letter symbol to make life simple for chemists. ...
Chapter 5 Atomic Structure and Periodic Table 2014
... Are these isotopes? If the blue spheres are the neutrons and the burgundy spheres are the protons, are these three items isotopes of an element? By the way, how many electrons does this atom have? ...
... Are these isotopes? If the blue spheres are the neutrons and the burgundy spheres are the protons, are these three items isotopes of an element? By the way, how many electrons does this atom have? ...
5Periodic Table of Elements WB
... group his cards according to team or position. Biologists classify all living organisms in a fivekingdom classification system, based on similar characteristics. In 1869 Dmitri Mendeleev, a Russian Chemist, published the first periodic table. It had eight columns and it contained blank spaces for el ...
... group his cards according to team or position. Biologists classify all living organisms in a fivekingdom classification system, based on similar characteristics. In 1869 Dmitri Mendeleev, a Russian Chemist, published the first periodic table. It had eight columns and it contained blank spaces for el ...
Review for Test
... 4. Describe the effects a stronger magnet would have had on the television image. __________________________________________________________________________________________________ __________________________________________________________________________________________________ ____________________ ...
... 4. Describe the effects a stronger magnet would have had on the television image. __________________________________________________________________________________________________ __________________________________________________________________________________________________ ____________________ ...
The Material World: An Introduction to Chemistry 1. Modern Model of
... explain why even the thin lines in an emission spectrum could be resolved into more fine lines, and they had to include the discovery of neutrons into their model. The atom is the smallest unit of an element that still behaves like the entire element, but that's not to say that the smaller parts do ...
... explain why even the thin lines in an emission spectrum could be resolved into more fine lines, and they had to include the discovery of neutrons into their model. The atom is the smallest unit of an element that still behaves like the entire element, but that's not to say that the smaller parts do ...
Nuclear Chemistry
... Nuclear Chemistry The strong force, also known as strong nuclear force, is stronger than the electrostatic repulsion. It holds the nucleus together. ...
... Nuclear Chemistry The strong force, also known as strong nuclear force, is stronger than the electrostatic repulsion. It holds the nucleus together. ...
File - 8th Grade Physical Science
... theory that all nature could be understood in terms of the movement of atomos. In ancient times, many elements were known, including C, S, Cu, Ag, Au, Fe, Sn, Sb and Pb. The names of most of these are from the Latin words. ...
... theory that all nature could be understood in terms of the movement of atomos. In ancient times, many elements were known, including C, S, Cu, Ag, Au, Fe, Sn, Sb and Pb. The names of most of these are from the Latin words. ...
Chapter 3-3—Parts of the Atom - Phoenix Union High School District
... Atoms have a center or core called a nucleus The nucleus contains 2 subatomic particles: Proton that has a positive charge Neutron that has a NEUTRAL charge Protons and neutrons together are called nucleons because they are in the nucleus The nucleus is surrounded by a cloud of very tiny particle ...
... Atoms have a center or core called a nucleus The nucleus contains 2 subatomic particles: Proton that has a positive charge Neutron that has a NEUTRAL charge Protons and neutrons together are called nucleons because they are in the nucleus The nucleus is surrounded by a cloud of very tiny particle ...
Distinguishing Between Atoms
... Since the mass of atoms is so increadibly small, a unit known as an atomic mass unit (amu) was defined to make the masses of atoms easier to work with. •The amu is defined by the carbon-12 isotope of carbon. •1 amu is equal to 1/12 the mass of the carbon-12 isotope. (roughly the mass of a neutron or ...
... Since the mass of atoms is so increadibly small, a unit known as an atomic mass unit (amu) was defined to make the masses of atoms easier to work with. •The amu is defined by the carbon-12 isotope of carbon. •1 amu is equal to 1/12 the mass of the carbon-12 isotope. (roughly the mass of a neutron or ...
Word List
... 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the periodic table. 1.12 I can write the names and symbols of selected transition metals, lanthanides and actinides (1B12B) on th ...
... 1.1 I can write the names and symbols of the elements in columns 1A – 4A on the periodic table. 1.5 I can write the names and symbols of the elements in columns 5A- 8A on the periodic table. 1.12 I can write the names and symbols of selected transition metals, lanthanides and actinides (1B12B) on th ...
Test Review with answer key and explanations
... Elements in the same group (vertical columns on the periodic table) have similar physical and chemical properties. The higher up on the periodic table something is, the lower the atomic mass. 2. The elements of group 18 are inert and unreactive because they have 8 valence electrons. This full orbita ...
... Elements in the same group (vertical columns on the periodic table) have similar physical and chemical properties. The higher up on the periodic table something is, the lower the atomic mass. 2. The elements of group 18 are inert and unreactive because they have 8 valence electrons. This full orbita ...
atomic number Protons, Neutrons, and Electrons
... • About 8 out of 10 chlorine atoms are chlorine-35. Two out of 10 are chlorine-37. ...
... • About 8 out of 10 chlorine atoms are chlorine-35. Two out of 10 are chlorine-37. ...
Blank Quiz - Fort Bend ISD
... Elements in the same group (vertical columns on the periodic table) have similar physical and chemical properties. The higher up on the periodic table something is, the lower the atomic mass. 2. The elements of group 18 are inert and unreactive because they have 8 valence electrons. This full orbita ...
... Elements in the same group (vertical columns on the periodic table) have similar physical and chemical properties. The higher up on the periodic table something is, the lower the atomic mass. 2. The elements of group 18 are inert and unreactive because they have 8 valence electrons. This full orbita ...
atoms - KMKunz
... other properties, but atoms of one element differ from the atoms of every other element • Compounds are formed when atoms of different elements unite in fixed proportions • A chemical reaction involves a rearrangement of atoms. No atoms are created, destroyed, or broken apart in a chemical reaction ...
... other properties, but atoms of one element differ from the atoms of every other element • Compounds are formed when atoms of different elements unite in fixed proportions • A chemical reaction involves a rearrangement of atoms. No atoms are created, destroyed, or broken apart in a chemical reaction ...
Neptunium
.png?width=300)
Neptunium is a chemical element with symbol Np and atomic number 93. A radioactive actinide metal, neptunium is the first transuranic element. Its position in the periodic table just after uranium, named after the planet Uranus, led to it being named after Neptune, the next planet beyond Uranus. A neptunium atom has 93 protons and 93 electrons, of which seven are valence electrons. Neptunium metal is silvery and tarnishes when exposed to air. The element occurs in three allotropic forms and it normally exhibits five oxidation states, ranging from +3 to +7. It is radioactive, pyrophoric, and can accumulate in bones, which makes the handling of neptunium dangerous.Although many false claims of its discovery were made over the years, the element was first synthesized by Edwin McMillan and Philip H. Abelson at the Berkeley Radiation Laboratory in 1940. Since then, most neptunium has been and still is produced by neutron irradiation of uranium in nuclear reactors. The vast majority is generated as a by-product in conventional nuclear power reactors. While neptunium itself has no commercial uses at present, it is widely used as a precursor for the formation of plutonium-238, used in radioisotope thermal generators. Neptunium has also been used in detectors of high-energy neutrons.The most stable isotope of neptunium, neptunium-237, is a by-product of nuclear reactors and plutonium production. It, and the isotope neptunium-239, are also found in trace amounts in uranium ores due to neutron capture reactions and beta decay.