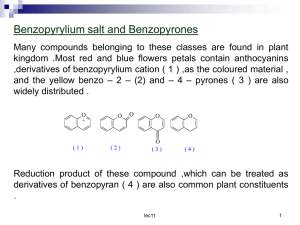
Post Lab Questions
... Your assignments are a reflection of you, your commitment to quality and your interest in the class. All assignments will be turned in on flat, smooth paper with no tears. Notebook paper will not have spiral notebook fuzz. All assignments are to be done in ink, blue or black only. Assignments should ...
... Your assignments are a reflection of you, your commitment to quality and your interest in the class. All assignments will be turned in on flat, smooth paper with no tears. Notebook paper will not have spiral notebook fuzz. All assignments are to be done in ink, blue or black only. Assignments should ...
Chemical Quantities and Aqueous Reactions
... molecules of octane burned. This numerical relationship between molecules can be extended to the amounts in moles as follows: The coefficients in a chemical reaction specify the relative amounts in moles of each of the substances involved in the reaction. In other words, from the equation, we know t ...
... molecules of octane burned. This numerical relationship between molecules can be extended to the amounts in moles as follows: The coefficients in a chemical reaction specify the relative amounts in moles of each of the substances involved in the reaction. In other words, from the equation, we know t ...
CSEC Chemistry Revision Guide Answers.indd
... Chlorine is above bromine in group VII so it has a smaller atomic radius and the attractive pull of the positive nucleus on the electron to be gained is stronger in chlorine. As a result, chlorine has a greater strength of oxidising power and readily takes electrons from the Br– ions causing them to ...
... Chlorine is above bromine in group VII so it has a smaller atomic radius and the attractive pull of the positive nucleus on the electron to be gained is stronger in chlorine. As a result, chlorine has a greater strength of oxidising power and readily takes electrons from the Br– ions causing them to ...
Exam Review Packet Table of Contents
... The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The radii of the alkali metal ions increase with increasing atomic number because: (i) the principle qua ...
... The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The radii of the alkali metal ions increase with increasing atomic number because: (i) the principle qua ...
Low-Temperature Alkaline pH Hydrolysis of Oxygen-Free
... characterization of a composition of salts in the subsurface ocean and cryolava. From this new and original chemical composition, a laboratory study of several hydrolyses of tholins was carried out. The results obtained show the formation of many organic compounds, among them, species identified onl ...
... characterization of a composition of salts in the subsurface ocean and cryolava. From this new and original chemical composition, a laboratory study of several hydrolyses of tholins was carried out. The results obtained show the formation of many organic compounds, among them, species identified onl ...
Worked solutions to the problems
... acid/base properties of CH4, NH3, H2S, H2O, HX NO reaction with O2 to form NO2 equilibrium between NO2 and N2O4 products of reaction of NO2 with water HNO2 and its salts are reductants HNO3 and its salts are oxidants N2H4 is a liquid and reductant there exists acids like H2N2O2, HN3 to remember, wha ...
... acid/base properties of CH4, NH3, H2S, H2O, HX NO reaction with O2 to form NO2 equilibrium between NO2 and N2O4 products of reaction of NO2 with water HNO2 and its salts are reductants HNO3 and its salts are oxidants N2H4 is a liquid and reductant there exists acids like H2N2O2, HN3 to remember, wha ...
study material(2014-15) class xii-chemistry
... Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials prepared by each participant were thoroughly reviewed by their co-particip ...
... Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials prepared by each participant were thoroughly reviewed by their co-particip ...
Textbook sample chapter
... change. Each gas behaves very slightly differently compared with other gases but, for most practical purposes, the differences are small enough to assume that gases all behave like an imaginary ‘ideal gas’. ...
... change. Each gas behaves very slightly differently compared with other gases but, for most practical purposes, the differences are small enough to assume that gases all behave like an imaginary ‘ideal gas’. ...
chemistry-resource
... Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials prepared by each participant were thoroughly reviewed by their co-particip ...
... Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials prepared by each participant were thoroughly reviewed by their co-particip ...
Practical Assessment of Sanitizers Steve Gray November
... • The sanitizing agent must result in irreversible damage referred to as microbial death • In the presence of 1,000,000 bacteria, 10 will survive • You now have a source for future contamination • Continued exposure to sub-lethal levels of sanitizer results in an increase in resistance • In essence ...
... • The sanitizing agent must result in irreversible damage referred to as microbial death • In the presence of 1,000,000 bacteria, 10 will survive • You now have a source for future contamination • Continued exposure to sub-lethal levels of sanitizer results in an increase in resistance • In essence ...
Exam 980415 - NTOU-Chem
... B) a ligand. C) a Lewis base. D) a Bronsted-Lowry base. E) insoluble in the solvent. Answer: A 42) For the phase transition from solid to liquid, ...
... B) a ligand. C) a Lewis base. D) a Bronsted-Lowry base. E) insoluble in the solvent. Answer: A 42) For the phase transition from solid to liquid, ...
BASIC CONCEPTS OF CHEMISTRY
... For acids and the bases f = 1 / m (where m is the basidity of an acid or acidity of a base). For oxides and salts f = 1 / n x V (where n is the number of metallic atoms in the compound, and V is the valency of the metal). Number of equivalents: n = m / МE (for any substance); n = V / VE (for gaseous ...
... For acids and the bases f = 1 / m (where m is the basidity of an acid or acidity of a base). For oxides and salts f = 1 / n x V (where n is the number of metallic atoms in the compound, and V is the valency of the metal). Number of equivalents: n = m / МE (for any substance); n = V / VE (for gaseous ...
Question Bank - Edudel.nic.in
... Why should a solution of a non volatile solute boil at a higher temperature? Explain with the help of a diagram. Derive the relationship between molar mass and elevation in boiling point. ...
... Why should a solution of a non volatile solute boil at a higher temperature? Explain with the help of a diagram. Derive the relationship between molar mass and elevation in boiling point. ...
4Chemical Quantities and Aqueous Reactions
... Without greenhouse gases in the atmosphere, more heat energy would escape, and Earth’s average temperature would be about 60 °F colder than it is now. The temperature outside of my office today would be below 0 °F, and even the sunniest U.S. cities would most likely be covered with snow. However, if ...
... Without greenhouse gases in the atmosphere, more heat energy would escape, and Earth’s average temperature would be about 60 °F colder than it is now. The temperature outside of my office today would be below 0 °F, and even the sunniest U.S. cities would most likely be covered with snow. However, if ...
CHAPTER 18
... In equilibrium, the concentrations of reactants and products remain constant. Suppose two substances, A and B, react to form products C and D. In turn, C and D react to produce A and B. Under appropriate conditions, equilibrium occurs for this reversible reaction. This hypothetical equilibrium react ...
... In equilibrium, the concentrations of reactants and products remain constant. Suppose two substances, A and B, react to form products C and D. In turn, C and D react to produce A and B. Under appropriate conditions, equilibrium occurs for this reversible reaction. This hypothetical equilibrium react ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.