Acid-Base Equilibria
... because of percent ionize is so small compared to initial conc of acid; remember 0.012-x=0.012. This wasn't the case for Kc or Kp problems so can't neglect in those type problems Material was developed by combining Janusa’s material with the lecture outline provided with Ebbing, D. D.; Gammon, S. D. ...
... because of percent ionize is so small compared to initial conc of acid; remember 0.012-x=0.012. This wasn't the case for Kc or Kp problems so can't neglect in those type problems Material was developed by combining Janusa’s material with the lecture outline provided with Ebbing, D. D.; Gammon, S. D. ...
A Biosensor Based on Immobilization of Horseradish Peroxidase in Chitosan Matrix Cross-linked with Glyoxal for Amperometric Determination of Hydrogen Peroxide
... copolymer of glucosamine and N-acetyglucosamine units linked by 1-4 glucosidic bonds, can be obtained by N-deacetylation of chitin, which is the second most abundant natural polymer [7]. Chitosan was selected as the matrix for immobilization of the enzyme because of an unusual combination of its pro ...
... copolymer of glucosamine and N-acetyglucosamine units linked by 1-4 glucosidic bonds, can be obtained by N-deacetylation of chitin, which is the second most abundant natural polymer [7]. Chitosan was selected as the matrix for immobilization of the enzyme because of an unusual combination of its pro ...
Practice Problems in Biomedical Organic Chemistry
... 10. (A) Aspirin, also known as acetylsalicylic acid, can be produced by reacting salicylic acid with acetic anhydride. In the reaction shown, a partially positive (δ+) carbon will be attacked by an anion (R-O-). Identify the partially positive carbons in the reactants and the products. ...
... 10. (A) Aspirin, also known as acetylsalicylic acid, can be produced by reacting salicylic acid with acetic anhydride. In the reaction shown, a partially positive (δ+) carbon will be attacked by an anion (R-O-). Identify the partially positive carbons in the reactants and the products. ...
Influence of Temperature on Electrical
... Influence of Temperature on Electrical Conductivity of Diluted Aqueous Solutions ...
... Influence of Temperature on Electrical Conductivity of Diluted Aqueous Solutions ...
quantitative chemistry
... interpreted. Namely, “a scientist chooses, imagines, does and describes”. Along with many other syllabuses, practical investigations are a requirement of IB Chemistry. Matter occupies space and has mass. It can be subdivided into mixtures and pure substances. Mixtures consist of a number of differen ...
... interpreted. Namely, “a scientist chooses, imagines, does and describes”. Along with many other syllabuses, practical investigations are a requirement of IB Chemistry. Matter occupies space and has mass. It can be subdivided into mixtures and pure substances. Mixtures consist of a number of differen ...
Document
... • so to describe solutions accurately, we must describe how much of each component is present we saw that with pure substances, we can describe them with a single name because all samples identical Tro, Chemistry: A Molecular Approach ...
... • so to describe solutions accurately, we must describe how much of each component is present we saw that with pure substances, we can describe them with a single name because all samples identical Tro, Chemistry: A Molecular Approach ...
TRO Chapter 4
... • so to describe solutions accurately, we must describe how much of each component is present we saw that with pure substances, we can describe them with a single name because all samples identical Tro, Chemistry: A Molecular Approach ...
... • so to describe solutions accurately, we must describe how much of each component is present we saw that with pure substances, we can describe them with a single name because all samples identical Tro, Chemistry: A Molecular Approach ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... • so to describe solutions accurately, we must describe how much of each component is present we saw that with pure substances, we can describe them with a single name because all samples identical Tro, Chemistry: A Molecular Approach ...
... • so to describe solutions accurately, we must describe how much of each component is present we saw that with pure substances, we can describe them with a single name because all samples identical Tro, Chemistry: A Molecular Approach ...
Basic chemistry help is available here for high school or college
... In many high schools and colleges the basic chemistry course is the one that causes most concern among students. With everything going right, chemistry can be a fun but challenging course. Under poor conditions, your first chemistry course can be a real spittin’, cussin’ nightmare. The study of chem ...
... In many high schools and colleges the basic chemistry course is the one that causes most concern among students. With everything going right, chemistry can be a fun but challenging course. Under poor conditions, your first chemistry course can be a real spittin’, cussin’ nightmare. The study of chem ...
evaluation copy
... material for any class he or she teaches. No part of these activities may be used or reproduced in any other manner without prior written permission of PASCO scientific, except in the case of brief quotations used in critical articles or reviews. SPARK Science Learning System, SPARKvue, PASCO Capsto ...
... material for any class he or she teaches. No part of these activities may be used or reproduced in any other manner without prior written permission of PASCO scientific, except in the case of brief quotations used in critical articles or reviews. SPARK Science Learning System, SPARKvue, PASCO Capsto ...
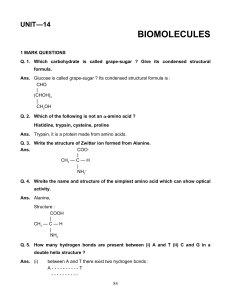
HOTS Worksheet
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
... Ans. The (— CO — NH —) amide bond in nylon gets hydrolysed. Q. 2. Fibres are of crystalline structure. Why ? Ans. Fibres have strong intermolecular forces of attraction which leads to close packing of their chains and impart crystalline structure. Q. 3. Which artificial polymer is present in bubble ...
Lab Manual (Eng. Medium)
... 1.5.2 Precautions (i) Slowly increase heating rate. (ii) Do not open the round bottom flask during heating. (iii) Stop distillation when a small amount of liquid is still left in the flask. Do not evaporate to dryness. (iv) Punice stones should be added in the beginning itself. (v) In case of organi ...
... 1.5.2 Precautions (i) Slowly increase heating rate. (ii) Do not open the round bottom flask during heating. (iii) Stop distillation when a small amount of liquid is still left in the flask. Do not evaporate to dryness. (iv) Punice stones should be added in the beginning itself. (v) In case of organi ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... • so to describe solutions accurately, we must describe how much of each component is present we saw that with pure substances, we can describe them with a single name because all samples identical Tro, Chemistry: A Molecular Approach ...
... • so to describe solutions accurately, we must describe how much of each component is present we saw that with pure substances, we can describe them with a single name because all samples identical Tro, Chemistry: A Molecular Approach ...
Chemistry - SSA Punjab
... and 20 secs. respectively. The molecular mass of A is 45. Find the molecular mass of B. ...
... and 20 secs. respectively. The molecular mass of A is 45. Find the molecular mass of B. ...
Alchemist`s Cookbook Student Part 2 (final)
... b) they go around in orbits, kind of like the solar system c) they live in houses, at least most of the time d) sometimes they disappear entirely 2. Electrons in atoms are often said to reside in "shells" around the nucleus. In general, more complex atoms have more electron shells. The number of she ...
... b) they go around in orbits, kind of like the solar system c) they live in houses, at least most of the time d) sometimes they disappear entirely 2. Electrons in atoms are often said to reside in "shells" around the nucleus. In general, more complex atoms have more electron shells. The number of she ...
Contents and Concepts Learning Objectives
... calcium oxalate dissolves. – Therefore, you expect calcium oxalate to be more soluble in acidic solution (low pH) than in pure ...
... calcium oxalate dissolves. – Therefore, you expect calcium oxalate to be more soluble in acidic solution (low pH) than in pure ...
Specification – AS/A Level Chemistry A
... This booklet contains OCR’s Advanced Subsidiary (AS) GCE and Advanced GCE specifications in Chemistry A for teaching from September 2013. This specification allows teachers to adopt a flexible approach to the delivery of AS and A Level Chemistry. The course has been designed to enable centres to del ...
... This booklet contains OCR’s Advanced Subsidiary (AS) GCE and Advanced GCE specifications in Chemistry A for teaching from September 2013. This specification allows teachers to adopt a flexible approach to the delivery of AS and A Level Chemistry. The course has been designed to enable centres to del ...
Solubility and Complex-ion Equilibria
... is 0.0025 M. If the concentration of oxalate ion is 1.0 x 10-7 M, do you expect calcium oxalate to precipitate? Ksp for calcium oxalate is 2.3 x 10-9. – The ion product quotient, Qc, is: ...
... is 0.0025 M. If the concentration of oxalate ion is 1.0 x 10-7 M, do you expect calcium oxalate to precipitate? Ksp for calcium oxalate is 2.3 x 10-9. – The ion product quotient, Qc, is: ...
chemistry - Brilliant Public School Sitamarhi
... in one unit cell of this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell is 5.46 × 10–8 cm in length. The density of the solid is 3.18 g cm–3. Use this i ...
... in one unit cell of this mineral there are 4 Ca2+ ions and 8F– ions and that Ca2+ ions are arranged in a fcc lattice. The F– ions fill all the tetrahedral holes in the fcc lattice of Ca2+ ions. The edge of the unit cell is 5.46 × 10–8 cm in length. The density of the solid is 3.18 g cm–3. Use this i ...
Contents and Concepts Learning Objectives
... Ag+, Cd2+, Cu2+, Fe2+, Fe3+, Ni2+, and Zn2+. • Complexing agents, called ligands, are Lewis bases. They include CN-, NH3, S2O32-, and OH. • In each case, an equilibrium is established, called the complex-ion formation equilibrium. ...
... Ag+, Cd2+, Cu2+, Fe2+, Fe3+, Ni2+, and Zn2+. • Complexing agents, called ligands, are Lewis bases. They include CN-, NH3, S2O32-, and OH. • In each case, an equilibrium is established, called the complex-ion formation equilibrium. ...
Contents and Concepts Learning Objectives
... form coordinate covalent bonds with molecules or anions having a lone pair of electrons. ...
... form coordinate covalent bonds with molecules or anions having a lone pair of electrons. ...
Acid
An acid (from the Latin acidus/acēre meaning sour) is a chemical substance whose aqueous solutions are characterized by a sour taste, the ability to turn blue litmus red, and the ability to react with bases and certain metals (like calcium) to form salts. Aqueous solutions of acids have a pH of less than 7. Non-aqueous acids are usually formed when an anion (negative ion) reacts with one or more positively charged hydrogen cations. A lower pH means a higher acidity, and thus a higher concentration of positive hydrogen ions in the solution. Chemicals or substances having the property of an acid are said to be acidic.There are three common definitions for acids: the Arrhenius definition, the Brønsted-Lowry definition, and the Lewis definition. The Arrhenius definition defines acids as substances which increase the concentration of hydrogen ions (H+), or more accurately, hydronium ions (H3O+), when dissolved in water. The Brønsted-Lowry definition is an expansion: an acid is a substance which can act as a proton donor. By this definition, any compound which can easily be deprotonated can be considered an acid. Examples include alcohols and amines which contain O-H or N-H fragments. A Lewis acid is a substance that can accept a pair of electrons to form a covalent bond. Examples of Lewis acids include all metal cations, and electron-deficient molecules such as boron trifluoride and aluminium trichloride.Common examples of acids include hydrochloric acid (a solution of hydrogen chloride which is found in gastric acid in the stomach and activates digestive enzymes), acetic acid (vinegar is a dilute solution of this liquid), sulfuric acid (used in car batteries), and tartaric acid (a solid used in baking). As these examples show, acids can be solutions or pure substances, and can be derived from solids, liquids, or gases. Strong acids and some concentrated weak acids are corrosive, but there are exceptions such as carboranes and boric acid.