![Neutral ionic liquid [BMIm]BF4 promoted highly selective](http://s1.studyres.com/store/data/017897985_1-047f9869d5604c115b21339541ccfffe-300x300.png)
Neutral ionic liquid [BMIm]BF4 promoted highly selective
... of tert-butanol by acetic anhydride using mesoporous Si-MCM41, clay and alumina supported metal Lewis acid, such as InCl3 and GaCl3 , as catalyst [9–11]. However, these metal species are toxic or expensive. Hence, there is a need to develop an environmentally benign method for the esterification of ...
... of tert-butanol by acetic anhydride using mesoporous Si-MCM41, clay and alumina supported metal Lewis acid, such as InCl3 and GaCl3 , as catalyst [9–11]. However, these metal species are toxic or expensive. Hence, there is a need to develop an environmentally benign method for the esterification of ...
Density, Viscosity, Solubility, and Diffusivity of N2O in Aqueous
... carrying out experiments on the absorption of N2O and CO2 in water in the temperature range 292-310 K, using different liquid stirrer speeds. The values of diffusion coefficient of N2O and CO2 in water obtained from the literature9 were used to determine the calibration factor, f. The f factor of th ...
... carrying out experiments on the absorption of N2O and CO2 in water in the temperature range 292-310 K, using different liquid stirrer speeds. The values of diffusion coefficient of N2O and CO2 in water obtained from the literature9 were used to determine the calibration factor, f. The f factor of th ...
Worksheet: Acid base problems - AP level
... 1) Use H-H Equation to determine required ratio of acetate to acid in solution: 5.000 = 4.752 + log [base] /[acid] log [base] /[acid] = 0.248 [base] /[acid] = 1.77 2) Determine molar amount of base required to get pH = 5.000 (for convenience, I'm going to use 1.00 L. I'll go to 250 mL at the end of ...
... 1) Use H-H Equation to determine required ratio of acetate to acid in solution: 5.000 = 4.752 + log [base] /[acid] log [base] /[acid] = 0.248 [base] /[acid] = 1.77 2) Determine molar amount of base required to get pH = 5.000 (for convenience, I'm going to use 1.00 L. I'll go to 250 mL at the end of ...
Questions
... State how propanone can be distinguished from propanal using infra-red spectra. You are not expected to give actual absorption values, but you should indicate the bonds in the molecules which would give rise to the distinguishing absorptions. ...
... State how propanone can be distinguished from propanal using infra-red spectra. You are not expected to give actual absorption values, but you should indicate the bonds in the molecules which would give rise to the distinguishing absorptions. ...
4 Acid Base Solutions
... Trichloroacetic acid (CCl3COOH) is the strongest acid because it has the most negative pKa (which is the largest Ka). b. Provide a reason using the molecular structure to explain why the acid in part a. is the strongest. The increased Cl polarizes the electrons away from and weakens the O−H bond, so ...
... Trichloroacetic acid (CCl3COOH) is the strongest acid because it has the most negative pKa (which is the largest Ka). b. Provide a reason using the molecular structure to explain why the acid in part a. is the strongest. The increased Cl polarizes the electrons away from and weakens the O−H bond, so ...
AP Chem Equations - Speedway High School
... equation. All reactions do not fit neatly into the five types of reactions that you learned in Chemistry I. ...
... equation. All reactions do not fit neatly into the five types of reactions that you learned in Chemistry I. ...
Organic Chemical Reactions
... These electrons are valence shell electrons and may be either bond or non-bond electrons. Motion of electron is shown using curved arrows. Depending on the number (either one or a pair) of electrons involved in a single motion, two types of curves have been defined (Figure 2). Mechanisms are extreme ...
... These electrons are valence shell electrons and may be either bond or non-bond electrons. Motion of electron is shown using curved arrows. Depending on the number (either one or a pair) of electrons involved in a single motion, two types of curves have been defined (Figure 2). Mechanisms are extreme ...
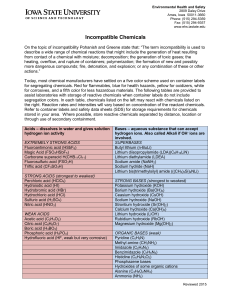
Incompatible Chemicals
... for corrosives, and a fifth color for less hazardous materials. The following tables are provided to assist laboratories with storage of reactive chemicals when container labels do not include segregation colors. In each table, chemicals listed on the left may react with chemicals listed on the righ ...
... for corrosives, and a fifth color for less hazardous materials. The following tables are provided to assist laboratories with storage of reactive chemicals when container labels do not include segregation colors. In each table, chemicals listed on the left may react with chemicals listed on the righ ...
local section exam
... pencil. Make a heavy, full mark, but no stray marks. If you decide to change an answer, erase the unwanted mark very carefully. ...
... pencil. Make a heavy, full mark, but no stray marks. If you decide to change an answer, erase the unwanted mark very carefully. ...
Hydrothermal Reactions from Sodium Hydrogen Carbonate to Phenol
... However, the information on how these organic molecules formed from the simplest inorganic molecules is still at a very beginning stage and defies clarity. Abiotic synthesis of organic compounds from CO2 under hydrothermal conditions has been proposed as a source of the precursor compounds from whic ...
... However, the information on how these organic molecules formed from the simplest inorganic molecules is still at a very beginning stage and defies clarity. Abiotic synthesis of organic compounds from CO2 under hydrothermal conditions has been proposed as a source of the precursor compounds from whic ...
Liquid–liquid extraction

Liquid–liquid extraction (LLE) consists in transferring one (or more) solute(s) contained in a feed solution to another immiscible liquid (solvent). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called raffinate.Liquid–liquid extraction also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid into another liquid phase. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment. This type of process is commonly performed after a chemical reaction as part of the work-up.The term partitioning is commonly used to refer to the underlying chemical and physical processes involved in liquid–liquid extraction, but on another reading may be fully synonymous with it. The term solvent extraction can also refer to the separation of a substance from a mixture by preferentially dissolving that substance in a suitable solvent. In that case, a soluble compound is separated from an insoluble compound or a complex matrix.Solvent extraction is used in nuclear reprocessing, ore processing, the production of fine organic compounds, the processing of perfumes, the production of vegetable oils and biodiesel, and other industries.Liquid–liquid extraction is possible in non-aqueous systems: In a system consisting of a molten metal in contact with molten salts, metals can be extracted from one phase to the other. This is related to a mercury electrode where a metal can be reduced, the metal will often then dissolve in the mercury to form an amalgam that modifies its electrochemistry greatly. For example, it is possible for sodium cations to be reduced at a mercury cathode to form sodium amalgam, while at an inert electrode (such as platinum) the sodium cations are not reduced. Instead, water is reduced to hydrogen. A detergent or fine solid can be used to stabilize an emulsion, or third phase.