summer fun - West Windsor-Plainsboro Regional School District
... The solubility of a solute is the amount that can be dissolved in a given quantity of solvent at a given temperature. For example, the solubility of lead (II) nitrate is 56 g/100 mL at 20oC. The solubilities of ionic solids in water vary over a wide range of values. For convenience, we divide compou ...
... The solubility of a solute is the amount that can be dissolved in a given quantity of solvent at a given temperature. For example, the solubility of lead (II) nitrate is 56 g/100 mL at 20oC. The solubilities of ionic solids in water vary over a wide range of values. For convenience, we divide compou ...
Types of Chemical Reactions
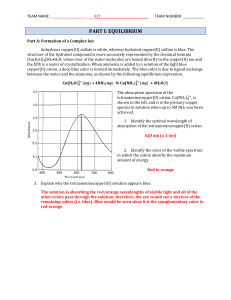
... The formation of a precipitate is a driving force for a chemical reaction. A precipitate is an insoluble solid that is formed when two aqueous solutions are mixed. We often separate the precipitate (ppt) from solution by filtration in what is called a gravimetric analysis. To identify the precipitat ...
... The formation of a precipitate is a driving force for a chemical reaction. A precipitate is an insoluble solid that is formed when two aqueous solutions are mixed. We often separate the precipitate (ppt) from solution by filtration in what is called a gravimetric analysis. To identify the precipitat ...
A Review of High School Chemistry
... Long about 1870, Mendeleev was inspired to put together a tabulation of the known elements in such a way as to describe in a periodic way their physical and chemical properties. The modern version of Mendeleev’s Periodic Table is an attractive addition to most science lecture halls—it is worth study ...
... Long about 1870, Mendeleev was inspired to put together a tabulation of the known elements in such a way as to describe in a periodic way their physical and chemical properties. The modern version of Mendeleev’s Periodic Table is an attractive addition to most science lecture halls—it is worth study ...
Solutions
... mol/m3, 8.2 mg/L, 5.9 mL/L, 55 mL/L, to name a few. And, fortunately, oxygen solubility is so small that the value is the same by amount of solution than by amount of solvent. The explanation of the above figures is that 78 mg/kg is equal to 78 mg/L for aqueous solutions where 1 L has 1 kg; it is al ...
... mol/m3, 8.2 mg/L, 5.9 mL/L, 55 mL/L, to name a few. And, fortunately, oxygen solubility is so small that the value is the same by amount of solution than by amount of solvent. The explanation of the above figures is that 78 mg/kg is equal to 78 mg/L for aqueous solutions where 1 L has 1 kg; it is al ...
Answers - Scioly.org
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
... 20. The student concludes that she has synthesized ethyl butanoate. Use evidence from the two experiments to support or to refute her claim. The peak of highest mass to charge ratio is approximately 116; therefore, the unknown molecule would have a molecular mass of 116. Ethyl butanoate has the chem ...
ICSE Board Class X Chemistry Board Paper – 2015
... affinity towards oxygen and so cannot be reduced by carbon. (Note: Error in the question. Zinc oxide can be reduced to zinc metal by using carbon, but aluminium oxide cannot be reduced by a reducing agent.) (ii) Carbon tetrachloride is made of individual covalently bonded molecules, CCl 4. In additi ...
... affinity towards oxygen and so cannot be reduced by carbon. (Note: Error in the question. Zinc oxide can be reduced to zinc metal by using carbon, but aluminium oxide cannot be reduced by a reducing agent.) (ii) Carbon tetrachloride is made of individual covalently bonded molecules, CCl 4. In additi ...
Past AP FRQ`s Linked to Text Chapters
... (d) Calculate the standard free energy of formation, Gf°, for butyric acid at 25 °C. Chapter 18: Electrochemistry The electrolysis of an aqueous solution of potassium iodide, KI, results in the formation of hydrogen gas at the cathode and iodine at the anode. A sample of 80.00 milliliters of a 0.15 ...
... (d) Calculate the standard free energy of formation, Gf°, for butyric acid at 25 °C. Chapter 18: Electrochemistry The electrolysis of an aqueous solution of potassium iodide, KI, results in the formation of hydrogen gas at the cathode and iodine at the anode. A sample of 80.00 milliliters of a 0.15 ...
Chemical Reactions
... Note the number of moles of gas on the left-hand side and the number of moles of gas on the righthand side. When the volume of the system is changed, the partial pressures of the gases change. If we were to decrease pressure by increasing volume, the equilibrium of the above reaction will shift to t ...
... Note the number of moles of gas on the left-hand side and the number of moles of gas on the righthand side. When the volume of the system is changed, the partial pressures of the gases change. If we were to decrease pressure by increasing volume, the equilibrium of the above reaction will shift to t ...
Liquid–liquid extraction

Liquid–liquid extraction (LLE) consists in transferring one (or more) solute(s) contained in a feed solution to another immiscible liquid (solvent). The solvent that is enriched in solute(s) is called extract. The feed solution that is depleted in solute(s) is called raffinate.Liquid–liquid extraction also known as solvent extraction and partitioning, is a method to separate compounds based on their relative solubilities in two different immiscible liquids, usually water and an organic solvent. It is an extraction of a substance from one liquid into another liquid phase. Liquid–liquid extraction is a basic technique in chemical laboratories, where it is performed using a variety of apparatus, from separatory funnels to countercurrent distribution equipment. This type of process is commonly performed after a chemical reaction as part of the work-up.The term partitioning is commonly used to refer to the underlying chemical and physical processes involved in liquid–liquid extraction, but on another reading may be fully synonymous with it. The term solvent extraction can also refer to the separation of a substance from a mixture by preferentially dissolving that substance in a suitable solvent. In that case, a soluble compound is separated from an insoluble compound or a complex matrix.Solvent extraction is used in nuclear reprocessing, ore processing, the production of fine organic compounds, the processing of perfumes, the production of vegetable oils and biodiesel, and other industries.Liquid–liquid extraction is possible in non-aqueous systems: In a system consisting of a molten metal in contact with molten salts, metals can be extracted from one phase to the other. This is related to a mercury electrode where a metal can be reduced, the metal will often then dissolve in the mercury to form an amalgam that modifies its electrochemistry greatly. For example, it is possible for sodium cations to be reduced at a mercury cathode to form sodium amalgam, while at an inert electrode (such as platinum) the sodium cations are not reduced. Instead, water is reduced to hydrogen. A detergent or fine solid can be used to stabilize an emulsion, or third phase.