CH 3
... chemical reactivity will be greater than the hydrocarbons having the same number of carbon atoms. • Saturated hydrocabons(Alkanes) can only give substitution and combustion reactions. • Alkenes and Alkynes are more reactive than alkanes due to existance of pi bonding.So they can also give addition r ...
... chemical reactivity will be greater than the hydrocarbons having the same number of carbon atoms. • Saturated hydrocabons(Alkanes) can only give substitution and combustion reactions. • Alkenes and Alkynes are more reactive than alkanes due to existance of pi bonding.So they can also give addition r ...
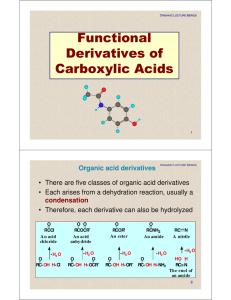
Functional Derivatives of Carboxylic Acids
... character toward attack by water (a weak nucleophile) to form a tetrahedral carbonyl addition intermediate – collapse of this intermediate gives the carboxylic acid and alcohol ...
... character toward attack by water (a weak nucleophile) to form a tetrahedral carbonyl addition intermediate – collapse of this intermediate gives the carboxylic acid and alcohol ...
Chapter 11: Reactions of Alcohols
... Unique Reactions of Diols (11.11) The dehydration of vicinal diols is unique simply because of the product that is generated. In this reaction known as the Pinacol Rearrangement, the product is no longer an alkene but rather a ketone. ...
... Unique Reactions of Diols (11.11) The dehydration of vicinal diols is unique simply because of the product that is generated. In this reaction known as the Pinacol Rearrangement, the product is no longer an alkene but rather a ketone. ...
Alcohols, Diols, and Thiols
... from wood—hence, the name wood alcohol. Now, most of the more than 10 billion lb of methanol used annually in the United States is synthetic, prepared by reduction of carbon monoxide with hydrogen. Carbon monoxide is normally made from methane. CO ...
... from wood—hence, the name wood alcohol. Now, most of the more than 10 billion lb of methanol used annually in the United States is synthetic, prepared by reduction of carbon monoxide with hydrogen. Carbon monoxide is normally made from methane. CO ...
Presentation
... the corresponding hydrocarbons due to (A) intermolecular hydrogen bonding (B) intramolecular hydrogen bonding (C) van der Waal’s forces of attraction (D) dipole – dipole interactions ...
... the corresponding hydrocarbons due to (A) intermolecular hydrogen bonding (B) intramolecular hydrogen bonding (C) van der Waal’s forces of attraction (D) dipole – dipole interactions ...
File - cpprashanths Chemistry
... ANS :KCN is predominantly ionic and provides cyanide ions in solution. However both carbon and nitrogen atoms are in a position to donate electron pairs, the attack takes place mainly through carbon atom and not through nitrogen atom since C-C bond is more stable the C-N bond. However, AgCN is main ...
... ANS :KCN is predominantly ionic and provides cyanide ions in solution. However both carbon and nitrogen atoms are in a position to donate electron pairs, the attack takes place mainly through carbon atom and not through nitrogen atom since C-C bond is more stable the C-N bond. However, AgCN is main ...
Ex 3 Aspirin
... Two different cyclooxygenase isozymes (COX-1 and COX-2) are responsible for prostaglandin synthesis. COX-1 is involved with the usual production of prostaglandins and plays a role as a ‘housekeeping enzyme’ in maintaining the lining of the stomach and in endothelial cells contributing to the normal ...
... Two different cyclooxygenase isozymes (COX-1 and COX-2) are responsible for prostaglandin synthesis. COX-1 is involved with the usual production of prostaglandins and plays a role as a ‘housekeeping enzyme’ in maintaining the lining of the stomach and in endothelial cells contributing to the normal ...
ch15[1].
... • Lactone: a cyclic ester • IUPAC: name the parent carboxylic acid, drop the suffix -ic acid, and add -olactone. • The location of the oxygen atom on the carbon chain is commonly indicated by a Greek letter. O 4-Butan olactone (A -lacton e) ...
... • Lactone: a cyclic ester • IUPAC: name the parent carboxylic acid, drop the suffix -ic acid, and add -olactone. • The location of the oxygen atom on the carbon chain is commonly indicated by a Greek letter. O 4-Butan olactone (A -lacton e) ...
word document
... Organic anhydrides contain the carbonyl group (CO). Organic anhydrides are formed by the condensation of original acids. Lactone, an internal cyclic monoester, is an anhydride derived from the hydroxyl and carboxyl radicals. In organic chemistry, most anhydride compounds are derived from correspondi ...
... Organic anhydrides contain the carbonyl group (CO). Organic anhydrides are formed by the condensation of original acids. Lactone, an internal cyclic monoester, is an anhydride derived from the hydroxyl and carboxyl radicals. In organic chemistry, most anhydride compounds are derived from correspondi ...
CH3
... 3. Cite some industrial uses of organic compounds ETHER. References: All organic chemistry book will do ...
... 3. Cite some industrial uses of organic compounds ETHER. References: All organic chemistry book will do ...
conversion of the OH group into a better leaving group, and
... Williamson ether synthesis can occur to form epoxides. ...
... Williamson ether synthesis can occur to form epoxides. ...
10 | Carbon: More Than Just Another Element
... With four electrons in its outer shell, carbon will form four bonds to reach an octet configuration. In contrast, the elements boron and nitrogen form three bonds in molecular compounds; oxygen forms two bonds; and hydrogen and the halogens form one bond. With a larger number of bonds comes the oppo ...
... With four electrons in its outer shell, carbon will form four bonds to reach an octet configuration. In contrast, the elements boron and nitrogen form three bonds in molecular compounds; oxygen forms two bonds; and hydrogen and the halogens form one bond. With a larger number of bonds comes the oppo ...
Chapter 24. Amines
... Azo-coupled products have extended conjugation that lead to low energy electronic transitions that occur in visible light (dyes) ...
... Azo-coupled products have extended conjugation that lead to low energy electronic transitions that occur in visible light (dyes) ...
Sodium tungstate dihydrate - Revista Virtual de Química
... f) Oxidation of organosulfur compounds The oxidation of organosulfur compounds is the most straightforward method for the preparation of sulfoxides and sulfones, important as commodity chemicals or pharmaceuticals. Noyori and co-workers reported an oxidative process using sodium tungstate dihydrate ...
... f) Oxidation of organosulfur compounds The oxidation of organosulfur compounds is the most straightforward method for the preparation of sulfoxides and sulfones, important as commodity chemicals or pharmaceuticals. Noyori and co-workers reported an oxidative process using sodium tungstate dihydrate ...
15alcpp - Knockhardy
... • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature rules for simple organic compounds • Recall the chemical properties of ...
... • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature rules for simple organic compounds • Recall the chemical properties of ...
No Slide Title
... • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature rules for simple organic compounds • Recall the chemical properties of ...
... • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature rules for simple organic compounds • Recall the chemical properties of ...
+ H 2 O(l) - Knockhardy
... • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature rules for simple organic compounds • Recall the chemical properties of ...
... • Recall the definition of a covalent bond • Recall the difference types of physical bonding • Be able to balance simple equations • Be able to write out structures for simple organic molecules • Understand the IUPAC nomenclature rules for simple organic compounds • Recall the chemical properties of ...
Amino acids
... • The nonpolar R-group fill up the interior of the folded protein and help give it its 3D shape. ...
... • The nonpolar R-group fill up the interior of the folded protein and help give it its 3D shape. ...
irm_ch25
... d. In Step 2, a keto group undergoes hydrogenation to form a secondary alcohol. 25.98 glucose, C8 fatty acid, sucrose, C14 fatty acid 25.99 The correct answer is b. Digestion of dietary triacylglycerols occurs to a small extent (10%) in the stomach. 25.100 a 25.101 The correct answer is c. The inter ...
... d. In Step 2, a keto group undergoes hydrogenation to form a secondary alcohol. 25.98 glucose, C8 fatty acid, sucrose, C14 fatty acid 25.99 The correct answer is b. Digestion of dietary triacylglycerols occurs to a small extent (10%) in the stomach. 25.100 a 25.101 The correct answer is c. The inter ...
In the data set I send you there are the compounds with some
... attached. This may be useful if you want to focalise your attention to certain classes MW: the molecular weight according to the EPA list (salt or hydrate). Since it is not correct to model for toxicity water or counter ions, descriptors have been calculated for the free compounds. As a consequenc ...
... attached. This may be useful if you want to focalise your attention to certain classes MW: the molecular weight according to the EPA list (salt or hydrate). Since it is not correct to model for toxicity water or counter ions, descriptors have been calculated for the free compounds. As a consequenc ...
19_03_05rw
... Normally, symmetrical anhydrides are used (both R groups the same). Reaction can be carried out in presence of pyridine (a base) or it can be catalyzed by acids. ...
... Normally, symmetrical anhydrides are used (both R groups the same). Reaction can be carried out in presence of pyridine (a base) or it can be catalyzed by acids. ...
Chapter-16A
... Carboxylic acids have significantly higher boiling points than other types of organic compounds of comparable molecular weight • they are polar compounds and form very strong intermolecular hydrogen bonds ...
... Carboxylic acids have significantly higher boiling points than other types of organic compounds of comparable molecular weight • they are polar compounds and form very strong intermolecular hydrogen bonds ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.







![ch15[1].](http://s1.studyres.com/store/data/008194241_2-0a33cfb98ac502873dac865380b726e0-300x300.png)