Functional Groups - Manasquan Public Schools
... changed to -amine. – Ethanamine is used to make plastics, pharmaceuticals, and pesticides. – Benzenamine is used to make furniture foam and some of the dyes that give clothes their colors. ...
... changed to -amine. – Ethanamine is used to make plastics, pharmaceuticals, and pesticides. – Benzenamine is used to make furniture foam and some of the dyes that give clothes their colors. ...
4.7 Organic chemistry
... Suggest the impact on fuels, feedstocks and petrochemicals of the depleting stocks of crude oil. Describe a life without oil or oil derived products. Look at the cultural and ...
... Suggest the impact on fuels, feedstocks and petrochemicals of the depleting stocks of crude oil. Describe a life without oil or oil derived products. Look at the cultural and ...
Organic Chemistry
... • More than 95% of known compounds are organic compounds (Carbon-containing) • Recall – Carbons can have 4 bonds – Nitrogen can have 3 bonds – Oxygen can have 2 bonds – Hydrogen can have 1 bond ...
... • More than 95% of known compounds are organic compounds (Carbon-containing) • Recall – Carbons can have 4 bonds – Nitrogen can have 3 bonds – Oxygen can have 2 bonds – Hydrogen can have 1 bond ...
Preparation and Physical Properties of Chitosan Benzoic Acid
... fiber-forming ability as well as biodegradability, biocompatibility, and abundance in Nature make them attractive materials in such application fields. However, although they have been excellent candidates for practical uses and commercialization, their uses in daily life or industry have been great ...
... fiber-forming ability as well as biodegradability, biocompatibility, and abundance in Nature make them attractive materials in such application fields. However, although they have been excellent candidates for practical uses and commercialization, their uses in daily life or industry have been great ...
Montmorillonite: An efficient, heterogeneous and
... physicochemical properties are similar their BET surface areas are quite different. K-10 has a higher surface area (about 250 m2g−1) compared to that of KSF (10 m2g−1) (Cativiela et al. 1993; Cseri et al., 1995). A catalyst is a chemical species that induces a chemical reaction to occur at a reasona ...
... physicochemical properties are similar their BET surface areas are quite different. K-10 has a higher surface area (about 250 m2g−1) compared to that of KSF (10 m2g−1) (Cativiela et al. 1993; Cseri et al., 1995). A catalyst is a chemical species that induces a chemical reaction to occur at a reasona ...
File
... • The spontaneous change that takes place in the optical rotation of and anomers of a sugar when they are dissolved in water. The optical rotations of the sugars change until they reach the same value. • the explanation for this mutarotation lies in the existence of an equilibrium between the op ...
... • The spontaneous change that takes place in the optical rotation of and anomers of a sugar when they are dissolved in water. The optical rotations of the sugars change until they reach the same value. • the explanation for this mutarotation lies in the existence of an equilibrium between the op ...
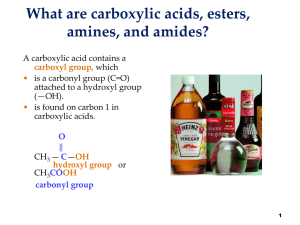
15: Carbonyl Compounds: Esters, Amides, and Related Molecules
... substitution reactions, you will see that nomenclature rules are signifcantly different for each class. Before we present systematic names for these compounds, you need to know that organic chemists informally use a form of common nomenclature based on the specific carboxylic acid that forms during ...
... substitution reactions, you will see that nomenclature rules are signifcantly different for each class. Before we present systematic names for these compounds, you need to know that organic chemists informally use a form of common nomenclature based on the specific carboxylic acid that forms during ...
Chapter 13. Alcohols, Diols, and Ethers
... • 1°-alcohol: stops at an aldehyde; 2°-alcohol: gives a ketone ...
... • 1°-alcohol: stops at an aldehyde; 2°-alcohol: gives a ketone ...
SMK RAJA PEREMPUAN, IPOH
... 15.4 Classification based on functional groups (general formula) 15.5 Nomenclature and structural formulae for each functional / radical group (refer to their trivial names) ...
... 15.4 Classification based on functional groups (general formula) 15.5 Nomenclature and structural formulae for each functional / radical group (refer to their trivial names) ...
15: Carbonyl Compounds: Esters, Amides, and Related Molecules
... that nomenclature rules are signifcantly different for each class. Before we present systematic names for these compounds, you need to know that organic chemists informally use a form of common nomenclature based on the specific carboxylic acid that forms during their hydrolysis (Figure 15.15 ). Fig ...
... that nomenclature rules are signifcantly different for each class. Before we present systematic names for these compounds, you need to know that organic chemists informally use a form of common nomenclature based on the specific carboxylic acid that forms during their hydrolysis (Figure 15.15 ). Fig ...
PDF - TU Darmstadt Chemie
... Carbohydrates serve as sources (sugars) and stores of energy (starch and glycogen); they also form a major portion of the supporting tissue of plants (cellulose) and of some animals (chitin in crustacea and insects); they play a basic role as part of the nucleic acids DNA and RNA. Other carbohydrate ...
... Carbohydrates serve as sources (sugars) and stores of energy (starch and glycogen); they also form a major portion of the supporting tissue of plants (cellulose) and of some animals (chitin in crustacea and insects); they play a basic role as part of the nucleic acids DNA and RNA. Other carbohydrate ...
Chapter 10
... • Thus the final reaction mixture will consist of varying ratios of RNH2, R2NH, R3N, and R4N+Cl–. • These ratios are difficult to control and, therefore, amines are avoided as nucleophiles in ...
... • Thus the final reaction mixture will consist of varying ratios of RNH2, R2NH, R3N, and R4N+Cl–. • These ratios are difficult to control and, therefore, amines are avoided as nucleophiles in ...
ALCOHOLS AND ETHERS
... 15-4A Acidic Properties Several important reactions of alcohols involve only the oxygen-hydrogen bond and leave the carbon-oxygen bond intact. An important example is salt formation with acids and bases. Alcohols, like water, are both weak bases and weak acids. The acid ionization constant (K,) of e ...
... 15-4A Acidic Properties Several important reactions of alcohols involve only the oxygen-hydrogen bond and leave the carbon-oxygen bond intact. An important example is salt formation with acids and bases. Alcohols, like water, are both weak bases and weak acids. The acid ionization constant (K,) of e ...
Chemistry Unit 1
... the downfall of the ‘life force’ theory. How do you explain organic compounds at present and define organic chemistry? The common feature of organic compounds is that they all contain the element carbon. Organic compounds are the compounds of carbon found in and derived from plants and animals. They ...
... the downfall of the ‘life force’ theory. How do you explain organic compounds at present and define organic chemistry? The common feature of organic compounds is that they all contain the element carbon. Organic compounds are the compounds of carbon found in and derived from plants and animals. They ...
Microsoft Word
... stereoselective Grignard addition of p-methoxyphenylmagnesium bromide to Nbenzylimine derived from (S)-2,3-O-isopropylidine glyceraldehyde followed by a single step conversion of N-Boc amine diol to oxazolidinone. Herein we report a short and stereoselective synthesis of (-)-cytoxazone 1, (+)-5-epi- ...
... stereoselective Grignard addition of p-methoxyphenylmagnesium bromide to Nbenzylimine derived from (S)-2,3-O-isopropylidine glyceraldehyde followed by a single step conversion of N-Boc amine diol to oxazolidinone. Herein we report a short and stereoselective synthesis of (-)-cytoxazone 1, (+)-5-epi- ...
DEVELOPMENT OF GREEN AND OF POLYMER
... Among the earliest known ruthenium based oxidizing agents was ruthenium tetroxide,2,4 which was successfully used for the oxidation of secondary alcohols to ketones. Though it was used to oxidize benzyl alcohol to benzaldehyde,4 aliphatic primary alcohols were oxidized to the corresponding carboxyli ...
... Among the earliest known ruthenium based oxidizing agents was ruthenium tetroxide,2,4 which was successfully used for the oxidation of secondary alcohols to ketones. Though it was used to oxidize benzyl alcohol to benzaldehyde,4 aliphatic primary alcohols were oxidized to the corresponding carboxyli ...
CHAPTER 11 BONDING AND MOLECULAR STRUCTURE:
... –The prefix cyclo- is added to the name of the alkane with the same number of carbons • When one substituent is present it is assumed to be at position one and is not numbered • When two alkyl substituents are present the one with alphabetical priority is given position 1 • Numbering continues to gi ...
... –The prefix cyclo- is added to the name of the alkane with the same number of carbons • When one substituent is present it is assumed to be at position one and is not numbered • When two alkyl substituents are present the one with alphabetical priority is given position 1 • Numbering continues to gi ...
INTRODUCTION TO ORGANIC CHEMISTRY
... RULE 3. Number the carbon chain of the parent compound starting with the end nearer to the double or triple bond. Use the smaller of the two numbers on the double- or triple-bonded carbon to indicate the position of the double or triple bond. Place this number in front of the alkene or alkyne name. ...
... RULE 3. Number the carbon chain of the parent compound starting with the end nearer to the double or triple bond. Use the smaller of the two numbers on the double- or triple-bonded carbon to indicate the position of the double or triple bond. Place this number in front of the alkene or alkyne name. ...
Chapter 10 Structure and Synthesis of Alcohols
... Addition of first equivalent of Grignard reagent: ...
... Addition of first equivalent of Grignard reagent: ...
Synthesis of Heterocycles from Anthranilic acid
... to form amine-imine complexed dianions. Capture of these intermediate with acyl halides normally gave aromatic quinazolines, a type of heterocyclic compounds that is considered to be highly interesting as scaffolds for development of new drugs. When the acyl halide was a tertiary 2-haloacyl halide, ...
... to form amine-imine complexed dianions. Capture of these intermediate with acyl halides normally gave aromatic quinazolines, a type of heterocyclic compounds that is considered to be highly interesting as scaffolds for development of new drugs. When the acyl halide was a tertiary 2-haloacyl halide, ...
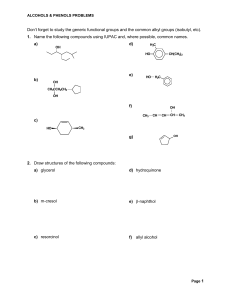
Don`t forget to study the generic functional groups and the common
... CH3 CH3 the type of reaction H3C ...
... CH3 CH3 the type of reaction H3C ...
Reactions of Alcohols
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
... The ZnCl2 coordinates to the hydroxyl oxygen, and this generates a far superior leaving group. Primary alcohols react in a similar fashion except the free cation is not generated, and the substitution is of S N2 ...
Phenols

In organic chemistry, phenols, sometimes called phenolics, are a class of chemical compounds consisting of a hydroxyl group (—OH) bonded directly to an aromatic hydrocarbon group. The simplest of the class is phenol, which is also called carbolic acid C6H5OH. Phenolic compounds are classified as simple phenols or polyphenols based on the number of phenol units in the molecule.Synonyms are arenols or aryl alcohols.Phenolic compounds are synthesized industrially; they also are produced by plants and microorganisms, with variation between and within species.Although similar to alcohols, phenols have unique properties and are not classified as alcohols (since the hydroxyl group is not bonded to a saturated carbon atom). They have higher acidities due to the aromatic ring's tight coupling with the oxygen and a relatively loose bond between the oxygen and hydrogen. The acidity of the hydroxyl group in phenols is commonly intermediate between that of aliphatic alcohols and carboxylic acids (their pKa is usually between 10 and 12).Loss of a positive hydrogen ion (H+) from the hydroxyl group of a phenol forms a corresponding negative phenolate ion or phenoxide ion, and the corresponding salts are called phenolates or phenoxides, although the term aryloxides is preferred according to the IUPAC Gold Book. Phenols can have two or more hydroxy groups bonded to the aromatic ring(s) in the same molecule. The simplest examples are the three benzenediols, each having two hydroxy groups on a benzene ring.Organisms that synthesize phenolic compounds do so in response to ecological pressures such as pathogen and insect attack, UV radiation and wounding. As they are present in food consumed in human diets and in plants used in traditional medicine of several cultures, their role in human health and disease is a subject of research.ref name=Klepacka Some phenols are germicidal and are used in formulating disinfectants. Others possess estrogenic or endocrine disrupting activity.