Name: Chem 22 Final exam Spring `00 What product is formed when
... e) addtion of a hydride ion and a proton more or less at the same time 18. Which of the following describes “reductive amination?” a) an aldehyde or a ketone + a tertiary amine + H2/zeolite b) an aldehyde or a ketone + ammonia or a primary or a secondary amine + ...
... e) addtion of a hydride ion and a proton more or less at the same time 18. Which of the following describes “reductive amination?” a) an aldehyde or a ketone + a tertiary amine + H2/zeolite b) an aldehyde or a ketone + ammonia or a primary or a secondary amine + ...
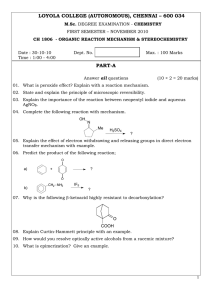
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034 PART-A
... 25. (a) Explain the addition-elimination reaction mechanism of a α, β-unsaturated ketone. ...
... 25. (a) Explain the addition-elimination reaction mechanism of a α, β-unsaturated ketone. ...
name Page 1 of 6 Multiple Choice. Choose the best answer for the
... they are stabilized by hyperconjugation. they cannot be observed, isolated or trapped. statements (a)-(d) are all true. ...
... they are stabilized by hyperconjugation. they cannot be observed, isolated or trapped. statements (a)-(d) are all true. ...
06 MC /08 MC /08 NMR
... Counting this cover sheet, there are a total of 9 pages- check to ensure you have all 9. All 9 pages must be turned in for grading. Use the backside of preceding pages for scratch paper. Any cheating will result in the dismissal from c/ass with an "F" grade. Please put your name or initial on each p ...
... Counting this cover sheet, there are a total of 9 pages- check to ensure you have all 9. All 9 pages must be turned in for grading. Use the backside of preceding pages for scratch paper. Any cheating will result in the dismissal from c/ass with an "F" grade. Please put your name or initial on each p ...
슬라이드 1
... The addition of halides to transition-metal species with low oxidation states is a common reaction in transition-metal chemistry and is called oxidative addition. The formal oxidation state of copper after addition is 3+. This step is followed by combination of two of the alkyl groups from c ...
... The addition of halides to transition-metal species with low oxidation states is a common reaction in transition-metal chemistry and is called oxidative addition. The formal oxidation state of copper after addition is 3+. This step is followed by combination of two of the alkyl groups from c ...
Exp 19 - Diphenylacetylene_2015
... aluminum block and stir until the stilbene has dissolved. In a test-tube, obtain approximately 1.2 mL of the 5% Br2 in DCM solution. This solution is approximately 1.0 M in Br2. Support this test tube in a small Erlenmeyer flask. Note: Bromine is toxic and can cause chemical burns. Wear gloves and k ...
... aluminum block and stir until the stilbene has dissolved. In a test-tube, obtain approximately 1.2 mL of the 5% Br2 in DCM solution. This solution is approximately 1.0 M in Br2. Support this test tube in a small Erlenmeyer flask. Note: Bromine is toxic and can cause chemical burns. Wear gloves and k ...
Classification of Halogen Derivatives
... Aryl halides are less reactive towards nucleophilic substitution reaction. Their low reactivity is attributed due to the following reasons: 1. Due to resonance, C-X bond has partial double bond character. 2. Stabilisation of the molecule by delocalisation of electrons. 3. (Instability of phenyl carb ...
... Aryl halides are less reactive towards nucleophilic substitution reaction. Their low reactivity is attributed due to the following reasons: 1. Due to resonance, C-X bond has partial double bond character. 2. Stabilisation of the molecule by delocalisation of electrons. 3. (Instability of phenyl carb ...
Organometallic Chemistry
... Dichloromethyl Methyl Ether Reaction • reaction of organoboranes with nucleophiles containing more than one leaving group results in multiple migrations. • Thus, on treatment of R3B with α,α-dichloromethyl methyl ether (DCME) in the presence of a sterically hindered base, such as Li-triethylmethoxi ...
... Dichloromethyl Methyl Ether Reaction • reaction of organoboranes with nucleophiles containing more than one leaving group results in multiple migrations. • Thus, on treatment of R3B with α,α-dichloromethyl methyl ether (DCME) in the presence of a sterically hindered base, such as Li-triethylmethoxi ...
Mechanism of Aldol Condensation
... carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone. ...
... carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone, followed by a dehydration to give a conjugated enone. ...
Excercises 6-10
... a. Give the full IUPAC name of the product. What is the overall reaction order of this reaction (and explain why)? b. According to which mechanism will the substitution reaction proceed when (R)-‐2-‐ iodo- ...
... a. Give the full IUPAC name of the product. What is the overall reaction order of this reaction (and explain why)? b. According to which mechanism will the substitution reaction proceed when (R)-‐2-‐ iodo- ...
Set 1 - ExamResults.net
... II. Answer Any FIVE of the following. ( Each questions carries 2 mark) 5 × 2 = 10 11. a) Give one example for paramagnetic substance. b) Which type of binding force existing in ice? 12. Write anodic and cathodic half-cell reactions taking place in Daniel cell. 13. Show that for first order reaction ...
... II. Answer Any FIVE of the following. ( Each questions carries 2 mark) 5 × 2 = 10 11. a) Give one example for paramagnetic substance. b) Which type of binding force existing in ice? 12. Write anodic and cathodic half-cell reactions taking place in Daniel cell. 13. Show that for first order reaction ...
Seminar_1 1. Classification and nomenclature of organic
... due to its high electronegativity. The first step in an E reaction is identical to the first step in an SN reaction. This is a problem because it leads to a mixture of products. The spontaneous formation of the carbocation is the slow step in both reactions. Once formed, the carbocation reacts rapid ...
... due to its high electronegativity. The first step in an E reaction is identical to the first step in an SN reaction. This is a problem because it leads to a mixture of products. The spontaneous formation of the carbocation is the slow step in both reactions. Once formed, the carbocation reacts rapid ...
Week 11 Problem Set (Solutions)
... that one of the OH groups leaves and then the leftover OH is turned into a ketone. The conversion of a diol into a ketone (with the number of carbons intact) is evidence of a Pinacol rearrangement. The Pinacol reaction occurs when a diol is subjected to a strong acid, like H2SO4. The more substitute ...
... that one of the OH groups leaves and then the leftover OH is turned into a ketone. The conversion of a diol into a ketone (with the number of carbons intact) is evidence of a Pinacol rearrangement. The Pinacol reaction occurs when a diol is subjected to a strong acid, like H2SO4. The more substitute ...
CH 3 Br + Nu
... 10. Which statement(s) is/are true of an E1 elimination? A) it is a two-step process and has the same first step as a SN1 mechanism B) it involves the formation of the carbocation from elimination of a good leaving group C) a common competing reaction is rearrangement of a less stable carbocation t ...
... 10. Which statement(s) is/are true of an E1 elimination? A) it is a two-step process and has the same first step as a SN1 mechanism B) it involves the formation of the carbocation from elimination of a good leaving group C) a common competing reaction is rearrangement of a less stable carbocation t ...
Organic Chemistry Unit Test
... b. Name each of your structures with a proper IUPAC name (5 marks) (write under ...
... b. Name each of your structures with a proper IUPAC name (5 marks) (write under ...
Quiz 3 – Aldehydes and Ketones 1 Which of the following reactions
... 7 You have two C6H10O ketones, I and II. Both are optically active, but I is racemized by treatment with base and II is not. Wolff-Kishner reduction of both ketones gives the same achiral hydrocarbon, formula C6H12. What reasonable structures may be assigned to I and II? A) I is 3-methyl-4-penten-2- ...
... 7 You have two C6H10O ketones, I and II. Both are optically active, but I is racemized by treatment with base and II is not. Wolff-Kishner reduction of both ketones gives the same achiral hydrocarbon, formula C6H12. What reasonable structures may be assigned to I and II? A) I is 3-methyl-4-penten-2- ...
Ch 26 C-C bond formation
... Organoboranes in Suzuki Reaction • Two types of organoboranes can be used in the Suzuki reaction: vinylboranes and arylboranes. • Vinylboranes, which have a boron atom bonded to a carbon– carbon double bond, are prepared by hydroboration using catecholborane, a commercially available reagent. • Hyd ...
... Organoboranes in Suzuki Reaction • Two types of organoboranes can be used in the Suzuki reaction: vinylboranes and arylboranes. • Vinylboranes, which have a boron atom bonded to a carbon– carbon double bond, are prepared by hydroboration using catecholborane, a commercially available reagent. • Hyd ...
effective: september 2003 curriculum guidelines
... complete mechanism of an allylic substitution reaction. 4. given a list of potential dienes and dienophiles, be able to predict the product of any combination (including stereochemical details) and the relative speed of the reaction. 5. give experimental evidence showing that benzene is resonance st ...
... complete mechanism of an allylic substitution reaction. 4. given a list of potential dienes and dienophiles, be able to predict the product of any combination (including stereochemical details) and the relative speed of the reaction. 5. give experimental evidence showing that benzene is resonance st ...
Arenes test - A-Level Chemistry
... In this question, one mark is available for the quality of use and organisation of scientific terms. Describe how benzene could be converted into nitrobenzene. State the reagents and conditions, give a balanced equation for each stage and show the structure of the product. ...
... In this question, one mark is available for the quality of use and organisation of scientific terms. Describe how benzene could be converted into nitrobenzene. State the reagents and conditions, give a balanced equation for each stage and show the structure of the product. ...
Grignard Reaction - This is Synthesis
... The Grignard reaction is a powerful, frequently used organometallic transformation to add important structural motifs to carbonyl compounds resulting in secondary or tertiary alcohols. Since usually the low boiling THF is used as a solvent, closed vessel conditions and elevated temperatures can sign ...
... The Grignard reaction is a powerful, frequently used organometallic transformation to add important structural motifs to carbonyl compounds resulting in secondary or tertiary alcohols. Since usually the low boiling THF is used as a solvent, closed vessel conditions and elevated temperatures can sign ...
Named Reactions Of Haloalkanes and haloarenes
... 7) Aldol condensation: Aldehydes and ketones having at least one α-hydrogen undergo a reaction in the presence of dilute alkali as catalyst to form βhydroxy aldehydes (aldol) or β-hydroxy ketones (ketol), respectively which readily undergoes condensation. OH ...
... 7) Aldol condensation: Aldehydes and ketones having at least one α-hydrogen undergo a reaction in the presence of dilute alkali as catalyst to form βhydroxy aldehydes (aldol) or β-hydroxy ketones (ketol), respectively which readily undergoes condensation. OH ...
NCEA Level 2 Chemistry (91165) 2012 Assessment Schedule
... correct. • In (a) reagent 4 (H2) is correct. • In (a) reagent 3 (PCl3 / PCl5 / SOCl2) is correct. ...
... correct. • In (a) reagent 4 (H2) is correct. • In (a) reagent 3 (PCl3 / PCl5 / SOCl2) is correct. ...
NCEA Level 2 Chemistry (91165) 2012
... correct. • In (a) reagent 4 (H2) is correct. • In (a) reagent 3 (PCl3 / PCl5 / SOCl2) is correct. ...
... correct. • In (a) reagent 4 (H2) is correct. • In (a) reagent 3 (PCl3 / PCl5 / SOCl2) is correct. ...
Diels–Alder reaction
.png?width=300)
The Diels–Alder reaction is an organic chemical reaction (specifically, a [4+2] cycloaddition) between a conjugated diene and a substituted alkene, commonly termed the dienophile, to form a substituted cyclohexene system. It was first described by Otto Paul Hermann Diels and Kurt Alder in 1928, for which work they were awarded the Nobel Prize in Chemistry in 1950. The Diels–Alder reaction is particularly useful in synthetic organic chemistry as a reliable method for forming 6-membered systems with good control over regio- and stereochemical properties. The underlying concept has also been applied to other π-systems, such as carbonyls and imines, to furnish the corresponding heterocycles, known as the hetero-Diels–Alder reaction. Diels–Alder reactions can be reversible under certain conditions; the reverse reaction is known as the retro-Diels–Alder reaction.