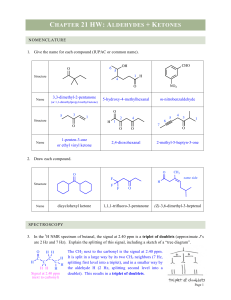
1990-Spring-Exam-2-student
... (CH3)2SO4, "PCC", CH2=CH-CH2-OH, acids, aldehydes, amines, alkenes, alkynes, ketones, thiols (R-SH); HCC-H, CHCl3, CCl4, CH2=CH-CH2OH, CH2I2, (C2H5)3B, NO, HO-CH2CH2OH, HS-CH2CH2SH, (CH3)3SiCl, CHBr3, CBr4 CF3CO3H (pertrifluoroacetic acid), CF3-SO2Cl (for "triflates"), CH2=CH-Br, CH2N2, CH2=C=O Cyc ...
... (CH3)2SO4, "PCC", CH2=CH-CH2-OH, acids, aldehydes, amines, alkenes, alkynes, ketones, thiols (R-SH); HCC-H, CHCl3, CCl4, CH2=CH-CH2OH, CH2I2, (C2H5)3B, NO, HO-CH2CH2OH, HS-CH2CH2SH, (CH3)3SiCl, CHBr3, CBr4 CF3CO3H (pertrifluoroacetic acid), CF3-SO2Cl (for "triflates"), CH2=CH-Br, CH2N2, CH2=C=O Cyc ...
Mock Exam One
... b.) In general, aldehydes are more reactive than ketones. c.) Nucleophilic addition to carbonyl groups can be catalyzed by acid or base. d.) Addition of a nucleophile to a carbonyl group changes the hybridization of the carbonyl carbon from sp3 to sp2. ...
... b.) In general, aldehydes are more reactive than ketones. c.) Nucleophilic addition to carbonyl groups can be catalyzed by acid or base. d.) Addition of a nucleophile to a carbonyl group changes the hybridization of the carbonyl carbon from sp3 to sp2. ...
Electrophilic Selenium Catalysis with Electrophilic N
... reagents ArSeX (X = Cl, Br, OTf, etc.) have been developed and widely applied in routine synthesis [1–9]. In general, the introduced selenium moiety was further modified via oxidative or reductive manner leading to the formation of non-selenium-containing products. This process was not green and was ...
... reagents ArSeX (X = Cl, Br, OTf, etc.) have been developed and widely applied in routine synthesis [1–9]. In general, the introduced selenium moiety was further modified via oxidative or reductive manner leading to the formation of non-selenium-containing products. This process was not green and was ...
9: Formation of Alkenes and Alkynes. Elimination Reactions
... elimination reactions of haloalkanes illustrate the fundamental features and mechanisms of many elimination reactions that form alkenes. Mechanisms for Elimination of H-X (9.1B) Elimination reactions of H-X occur primarily by either an E1 or E2 mechanism. In a number of ways, these mechanisms are si ...
... elimination reactions of haloalkanes illustrate the fundamental features and mechanisms of many elimination reactions that form alkenes. Mechanisms for Elimination of H-X (9.1B) Elimination reactions of H-X occur primarily by either an E1 or E2 mechanism. In a number of ways, these mechanisms are si ...
lecture 6 oxidative addition
... OXIDATIVE ADDITION – RADICAL MECHANISM • Radical mechanisms in oxidative additions were recognized later than the SN2 and the concerted processes. • They are normally photoinitiated. • A troublesome feature of these reactions is that minor changes in the structure of the substrate, the complex, or ...
... OXIDATIVE ADDITION – RADICAL MECHANISM • Radical mechanisms in oxidative additions were recognized later than the SN2 and the concerted processes. • They are normally photoinitiated. • A troublesome feature of these reactions is that minor changes in the structure of the substrate, the complex, or ...
Chapter Seven PPT
... • Unsaturated Compounds (Less than Maximum H Atoms) • Alkenes also Referred to as Olefins • Properties Similar to those of Corresponding Alkanes • Slightly Soluble in Water • Dissolve Readily in Nonpolar or Low Polarity Solvents • Densities of Alkenes and Alkynes Less than Water ...
... • Unsaturated Compounds (Less than Maximum H Atoms) • Alkenes also Referred to as Olefins • Properties Similar to those of Corresponding Alkanes • Slightly Soluble in Water • Dissolve Readily in Nonpolar or Low Polarity Solvents • Densities of Alkenes and Alkynes Less than Water ...
Copper-catalysed selective hydroamination reactions of alkynes Please share
... In addition to enamine synthesis, we speculated on the possibility that conditions could be developed to convert alkynes to enantioenriched α-branched alkylamines and/or linear alkylamines in one synthetic operation (Fig. 1b, B). Such cascade processes are highly desirable in organic synthesis, sinc ...
... In addition to enamine synthesis, we speculated on the possibility that conditions could be developed to convert alkynes to enantioenriched α-branched alkylamines and/or linear alkylamines in one synthetic operation (Fig. 1b, B). Such cascade processes are highly desirable in organic synthesis, sinc ...
Novel Transition Metal-Catalysed Syntheses of Carboxylic Acid
... Consecutive reactions differ from tandem reactions in that another reagent, mediator or catalyst is added after the first transformation without isolation of the first formed product; the subsequent reaction steps then lead to the final product. Whilst some operational simplicity is lost, such react ...
... Consecutive reactions differ from tandem reactions in that another reagent, mediator or catalyst is added after the first transformation without isolation of the first formed product; the subsequent reaction steps then lead to the final product. Whilst some operational simplicity is lost, such react ...
General and Selective Synthesis of (Z)-3
... 1-heptyne (1a) with CO (1 atm) and MeOH (2a, 0.6 mL) in the presence of 5 mol % of PdBr2 and benzene (10 mL) when the loadings of CuBr2 were increased to 5 equiv (entry 2). Interestingly, a 16% yield of 3 as a mixture of (Z)- and (E)-isomers was obtained by decreasing the amount of MeOH to 0.2 mL (e ...
... 1-heptyne (1a) with CO (1 atm) and MeOH (2a, 0.6 mL) in the presence of 5 mol % of PdBr2 and benzene (10 mL) when the loadings of CuBr2 were increased to 5 equiv (entry 2). Interestingly, a 16% yield of 3 as a mixture of (Z)- and (E)-isomers was obtained by decreasing the amount of MeOH to 0.2 mL (e ...
File
... strong signal at 1691 cm-1. Both signals represent vibration of the same kind of bond. Explain why the absorption in 2-cyclohexenone is at a lower wavenumber, including resonance structures. Both signals represent the IR stretching of the C=O bonds. 2-cyclohexenone has a lower wavenumber absorbance, ...
... strong signal at 1691 cm-1. Both signals represent vibration of the same kind of bond. Explain why the absorption in 2-cyclohexenone is at a lower wavenumber, including resonance structures. Both signals represent the IR stretching of the C=O bonds. 2-cyclohexenone has a lower wavenumber absorbance, ...
9: Formation of Alkenes and Alkynes. Elimination Reactions
... The C-A and C-B bonds break in the elimination reaction, and a second bond forms between the two C's to form a C=C bond. "A-B" in Figure 9.01 may not be an actual reaction product, but we show it this way in this general example in order to keep the chemical bonds on both sides of the equation in ba ...
... The C-A and C-B bonds break in the elimination reaction, and a second bond forms between the two C's to form a C=C bond. "A-B" in Figure 9.01 may not be an actual reaction product, but we show it this way in this general example in order to keep the chemical bonds on both sides of the equation in ba ...
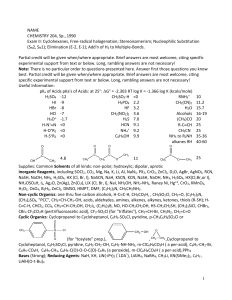
IOSR Journal of Applied Chemistry (IOSR-JAC)
... Michael addition reaction from suitably substituted alkene (31) using Fe(NO3)3 as catalyst (Scheme 12).60 This method was exclusively used for synthesis of pyrrolidine. An intramolecular ring expansion strategy of epoxide has been utilised using Fe-NHC catalytic condition, where FeCl2 was used as pr ...
... Michael addition reaction from suitably substituted alkene (31) using Fe(NO3)3 as catalyst (Scheme 12).60 This method was exclusively used for synthesis of pyrrolidine. An intramolecular ring expansion strategy of epoxide has been utilised using Fe-NHC catalytic condition, where FeCl2 was used as pr ...
university of london thesis
... industry, has almost invariably been selected as an excellent starting material for the production o f I-carvone 3. In essence, starting from this abundant raw material 1, endocyclic allylic oxidation with double bond transposition is required ...
... industry, has almost invariably been selected as an excellent starting material for the production o f I-carvone 3. In essence, starting from this abundant raw material 1, endocyclic allylic oxidation with double bond transposition is required ...
top organomet chem-2006-19-207 pauson
... ies support this mechanism while it explains the regio- and stereochemical results of numerous examples. Thus, Nakamura [53] and Milet and Gimbert [54] have performed high-level theoretical calculations on the cobaltacycle formation step, showing that the insertion of the olefin is the critical stere ...
... ies support this mechanism while it explains the regio- and stereochemical results of numerous examples. Thus, Nakamura [53] and Milet and Gimbert [54] have performed high-level theoretical calculations on the cobaltacycle formation step, showing that the insertion of the olefin is the critical stere ...
1 THE BARTON-McCOMBIE REACTION STUART W. McCOMBIE 28
... Deoxygenations of alcohols, i.e. processes that replace a hydroxyl group with hydrogen at a saturated carbon, find applications in both total synthesis and the systematic modification of natural products. They may also be employed to introduce deuterium or tritium in a site-specific manner. Reductiv ...
... Deoxygenations of alcohols, i.e. processes that replace a hydroxyl group with hydrogen at a saturated carbon, find applications in both total synthesis and the systematic modification of natural products. They may also be employed to introduce deuterium or tritium in a site-specific manner. Reductiv ...
Manganese-Catalyzed Carbonylation of Alkyl Iodides
... formation have been developed. 1' 2 Although highly reactive in solution, arynes can be stabilized by electron rich metal fragments. This stabilization can be attributed to several factors: 1) the aryne is less strained due to the back bonding of the metal which decreases the order of the triple bon ...
... formation have been developed. 1' 2 Although highly reactive in solution, arynes can be stabilized by electron rich metal fragments. This stabilization can be attributed to several factors: 1) the aryne is less strained due to the back bonding of the metal which decreases the order of the triple bon ...
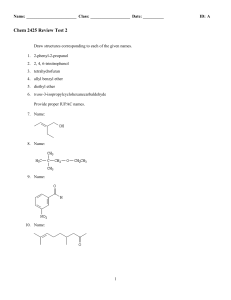
Chem 2425-Test 2 Review
... Epoxides are synthesized industrially in one step by silver oxide air oxidation of ethylene and on a laboratory scale in one step by treating an alkene with m-chloroperoxybenzoic acid. An alternative two step process converts alkenes to halohydrins, which are converted by treatment with base to epox ...
... Epoxides are synthesized industrially in one step by silver oxide air oxidation of ethylene and on a laboratory scale in one step by treating an alkene with m-chloroperoxybenzoic acid. An alternative two step process converts alkenes to halohydrins, which are converted by treatment with base to epox ...
Chloroperbenzoic_aci..
... Diastereoselective Epoxidation of Cyclic Alkenes. π-Facial stereoselectivity (75% anti) is observed in the epoxidation of the allyl ether (10a) since reagent approach from the α-face is blocked by the allylic substituent; a higher diastereoselectivity (90% anti epoxidation) is observed when the bulk ...
... Diastereoselective Epoxidation of Cyclic Alkenes. π-Facial stereoselectivity (75% anti) is observed in the epoxidation of the allyl ether (10a) since reagent approach from the α-face is blocked by the allylic substituent; a higher diastereoselectivity (90% anti epoxidation) is observed when the bulk ...
Ch 7 - Practice problem (Answers)
... Chapter 7 Structure and Preparation of Alkenes - Elimination Reactions: Answers ...
... Chapter 7 Structure and Preparation of Alkenes - Elimination Reactions: Answers ...
lecture 7 reductive eliminations
... OXIDATIVE ADDITION – CONCERTED MECHANISM • Concerted, or three‐center, oxidative addition is really an associative reaction in which the incoming ligand first binds as a σ complex and then undergoes bond breaking as a result of strong back donation from the metal into the * orbital. • Non‐polar re ...
... OXIDATIVE ADDITION – CONCERTED MECHANISM • Concerted, or three‐center, oxidative addition is really an associative reaction in which the incoming ligand first binds as a σ complex and then undergoes bond breaking as a result of strong back donation from the metal into the * orbital. • Non‐polar re ...
- Wiley Online Library
... require low loadings of iridium. The methodology is operationally very simple and can be scaled up. On-going mechanistic investigations should also contribute to the future development of Ir-catalyzed reactions for the formation of carbon–heteroatom bonds, and will be reported in due course. ...
... require low loadings of iridium. The methodology is operationally very simple and can be scaled up. On-going mechanistic investigations should also contribute to the future development of Ir-catalyzed reactions for the formation of carbon–heteroatom bonds, and will be reported in due course. ...
4.7 Preparation of Alkyl Halides from Alcohols and Hydrogen
... The product of this step is a carbocation. It is an intermediate in the overall process. ...
... The product of this step is a carbocation. It is an intermediate in the overall process. ...
- Sacramento - California State University
... 7. Figure 7: Ligand used for the asymmetric sulfoxidation of tert-butyl disulfide. ........ 17 8. Figure 8: Ligands for the kinetic resolution of ethyl mandalate (racemic) ................. 18 9. Figure 9: Ligands used in Jacobsen’s Epoxidation ..................................................... 2 ...
... 7. Figure 7: Ligand used for the asymmetric sulfoxidation of tert-butyl disulfide. ........ 17 8. Figure 8: Ligands for the kinetic resolution of ethyl mandalate (racemic) ................. 18 9. Figure 9: Ligands used in Jacobsen’s Epoxidation ..................................................... 2 ...
Vinylcyclopropane rearrangement

The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.Due to its ability to form cyclopentene rings the vinylcyclopropane rearrangement has served several times as a key reaction in complex natural product synthesis.