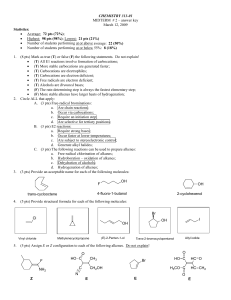
Chem 240 - Napa Valley College
... between phenyl magnesium bromide and benzaldehyde. To make sure his yield was good he added twice as much benzaldehyde as Grignard reagent and got a lot of white crystalline product. When he analyzed his product he found that he had not made diphenyl methanol, but diphenyl methanal (also called benz ...
... between phenyl magnesium bromide and benzaldehyde. To make sure his yield was good he added twice as much benzaldehyde as Grignard reagent and got a lot of white crystalline product. When he analyzed his product he found that he had not made diphenyl methanol, but diphenyl methanal (also called benz ...
Mannich Reaction - SUST Repository
... 1).52 Such molecules are useful in the treatment of hypertension. 1,2,4-Triazole derived Mannich bases exhibited anticancer activity.53 The isothiazolopyridine derived Mannich bases (16) were found to be 2 to 10 times more potent than the reference drug acetylsalicylic acid (chart 1).54 The Mannich ...
... 1).52 Such molecules are useful in the treatment of hypertension. 1,2,4-Triazole derived Mannich bases exhibited anticancer activity.53 The isothiazolopyridine derived Mannich bases (16) were found to be 2 to 10 times more potent than the reference drug acetylsalicylic acid (chart 1).54 The Mannich ...
Introduction to Nanochemistry
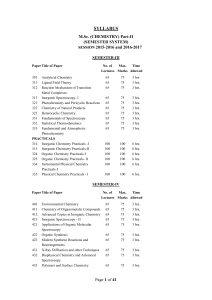
... INSTRUCTIONS FOR THE CANDIDATES Candidates are required to attempt five questions selecting two questions from each of Section A & B and entire Section C. Section - A ...
... INSTRUCTIONS FOR THE CANDIDATES Candidates are required to attempt five questions selecting two questions from each of Section A & B and entire Section C. Section - A ...
No Slide Title
... • Need a nonpolar, nonreactive solvent to dissolve argenine without interfering with the reaction. • Methyl Sulfoxide NOT efficient as a solvent for this reaction due to its exothermicity. – i.e (Broken Manifold and intense sulfur scent) • Length of complete reaction and temperature requirements are ...
... • Need a nonpolar, nonreactive solvent to dissolve argenine without interfering with the reaction. • Methyl Sulfoxide NOT efficient as a solvent for this reaction due to its exothermicity. – i.e (Broken Manifold and intense sulfur scent) • Length of complete reaction and temperature requirements are ...
dr.ebtehal Lec3
... Asian flush, Asian glow, among others) is a condition in which an individual's face or body experiences flushes or blotches as a result of an accumulation of acetaldehyde, a metabolic byproduct of the catabolic metabolism of alcohol. It is commonly thought that the flush reaction is caused by an ina ...
... Asian flush, Asian glow, among others) is a condition in which an individual's face or body experiences flushes or blotches as a result of an accumulation of acetaldehyde, a metabolic byproduct of the catabolic metabolism of alcohol. It is commonly thought that the flush reaction is caused by an ina ...
Learning materials
... 3. SN1 reaction 4. SN2 reaction 5. E1, E2 reactions 6. Applications of alkyl halides 7. Acidity and basicity of alcohols 8. Dehydration of alcohols 9. Synthesis of ethers 10. Solvents in organic chemistry ...
... 3. SN1 reaction 4. SN2 reaction 5. E1, E2 reactions 6. Applications of alkyl halides 7. Acidity and basicity of alcohols 8. Dehydration of alcohols 9. Synthesis of ethers 10. Solvents in organic chemistry ...
M.Sc. II - Punjabi University
... will have two questions from the respective sections of the syllabus and will carry 12 marks each. Section E will consist of 8 short-answer questions (two from each section) and will be of 1½ marks each. INSTRUCTIONS FOR THE CANDIDATES Candidates are required to attempt five questions selecting one ...
... will have two questions from the respective sections of the syllabus and will carry 12 marks each. Section E will consist of 8 short-answer questions (two from each section) and will be of 1½ marks each. INSTRUCTIONS FOR THE CANDIDATES Candidates are required to attempt five questions selecting one ...
synthesis, chemistry and optical resol
... in 1 ,3-propanediamine.17 All procedures gave rise to mixtures containing principally trisubstituted alkenes 1915at relative rates of roughly 2:l in favor of the cis isomer 12c. Nakazaki and co-workers recently obtained the first optically active betweenanene, "(-)-( R)-Dz-bicyclo[ 8.8 .O]octadec- 1 ...
... in 1 ,3-propanediamine.17 All procedures gave rise to mixtures containing principally trisubstituted alkenes 1915at relative rates of roughly 2:l in favor of the cis isomer 12c. Nakazaki and co-workers recently obtained the first optically active betweenanene, "(-)-( R)-Dz-bicyclo[ 8.8 .O]octadec- 1 ...
Organocatalysed asymmetric Mannich reactions
... provided the desired Mannich product in enantiopure form. As an example, reaction of L-proline, p-nitrobenzaldehyde (4), acetone (5) and p-anisidine (6) in DMF led to the desired Mannich adduct 10 in 50% yield with an ee of 94% (Scheme 2). This proceeds via the chiral proline-derived enamine 8, whic ...
... provided the desired Mannich product in enantiopure form. As an example, reaction of L-proline, p-nitrobenzaldehyde (4), acetone (5) and p-anisidine (6) in DMF led to the desired Mannich adduct 10 in 50% yield with an ee of 94% (Scheme 2). This proceeds via the chiral proline-derived enamine 8, whic ...
Ir-catalysed formation of C− F bonds. From allylic alcohols to α
... were obtained (i.e. no difluorinated ketones), under any of the reaction conditions. Since SelectF in THF/water mixtures successfully produced only one constitutional isomer of the product (i.e. 2a 0 was not formed), further screening on this system was performed (Table 2). The best results were obta ...
... were obtained (i.e. no difluorinated ketones), under any of the reaction conditions. Since SelectF in THF/water mixtures successfully produced only one constitutional isomer of the product (i.e. 2a 0 was not formed), further screening on this system was performed (Table 2). The best results were obta ...
Oxidation of Benzyl Ethers to Benzoate Esters Using a Novel
... enthalpically favored H-atom abstraction (Feray et. al., 2001) from the benzylic site produces the stable α-alkoxy radical, 5, which can be oxidized to benzaldehyde in one of two ways. A single electron transfer (SET) from 5 to the odd-electron species, 6, produces the benzylic carbocation 8 which c ...
... enthalpically favored H-atom abstraction (Feray et. al., 2001) from the benzylic site produces the stable α-alkoxy radical, 5, which can be oxidized to benzaldehyde in one of two ways. A single electron transfer (SET) from 5 to the odd-electron species, 6, produces the benzylic carbocation 8 which c ...
Chapter Seven - U of L Class Index
... H Carbocation rearrangments are often promoted by the presence of Lewis Acids. In this case, the intermediates are said to be “carbocation-like” if not carbocations. ...
... H Carbocation rearrangments are often promoted by the presence of Lewis Acids. In this case, the intermediates are said to be “carbocation-like” if not carbocations. ...
Development of New Synthetic Routes to Organoboronates by Catalytic Allylic Substitution and
... To access simple boronates such as allyl (R1=H, R2=H, Scheme 9) and crotyl (R1=Me, R2=H, Scheme 9) species, this method has the advantage of using readily available and inexpensive starting materials. However, these reactive species are known to undergo facile metallotropic rearrangements,109-111 wh ...
... To access simple boronates such as allyl (R1=H, R2=H, Scheme 9) and crotyl (R1=Me, R2=H, Scheme 9) species, this method has the advantage of using readily available and inexpensive starting materials. However, these reactive species are known to undergo facile metallotropic rearrangements,109-111 wh ...
13-Elimination Reactions
... The two mechanistic pathways that 1,2-elimination reactions take are designated as E1 and E2. The E stands for the elimination pathway, and the number describes the kinetics of the reaction— either unimolecular or bimolecular. E1 and E2 reactions relate closely to SN1 and SN2 reactions, so both a su ...
... The two mechanistic pathways that 1,2-elimination reactions take are designated as E1 and E2. The E stands for the elimination pathway, and the number describes the kinetics of the reaction— either unimolecular or bimolecular. E1 and E2 reactions relate closely to SN1 and SN2 reactions, so both a su ...
$doc.title
... Basicity of Arylamines • The N lone-‐pair electrons in arylamines are delocalized by interacHon with the aromaHc ring π electron system and are less able to accept H+ than are alkylamines ...
... Basicity of Arylamines • The N lone-‐pair electrons in arylamines are delocalized by interacHon with the aromaHc ring π electron system and are less able to accept H+ than are alkylamines ...
Latest Publication (still not complete)
... cyclization processes, concentrating on the synthesis of three- to seven-membered ring containing products. However, in addition to this, the structure and bonding of carbene complexes is also discussed. In particular, an analysis of information gained through computational analysis is provided. Suc ...
... cyclization processes, concentrating on the synthesis of three- to seven-membered ring containing products. However, in addition to this, the structure and bonding of carbene complexes is also discussed. In particular, an analysis of information gained through computational analysis is provided. Suc ...
Alkyl Aryl Ether Bond Formation with PhenoFluor
... bond formation is appealing and orthogonal to other crosscoupling approaches. The Mitsunobu reaction has been developed for this purpose, and a large substrate scope has been demonstrated.[6, 7] However, several substrate classes, such as salicylaldehydes, are not tolerated, and general alcohol–alco ...
... bond formation is appealing and orthogonal to other crosscoupling approaches. The Mitsunobu reaction has been developed for this purpose, and a large substrate scope has been demonstrated.[6, 7] However, several substrate classes, such as salicylaldehydes, are not tolerated, and general alcohol–alco ...
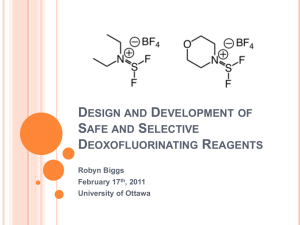
Design and Development of Safe and Selective Deoxofluorinating
... Highly toxic and corrosive Typically requires high temperatures (>100°C) Low selectivity ...
... Highly toxic and corrosive Typically requires high temperatures (>100°C) Low selectivity ...
Lecture - Ch 18
... Rearrangement • Takes place in a single step through a pericyclic mechanism – Reorganization of bonding electrons occurs in a six-membered, cyclic transition state ...
... Rearrangement • Takes place in a single step through a pericyclic mechanism – Reorganization of bonding electrons occurs in a six-membered, cyclic transition state ...
Aromatic Compounds
... Rings: The Friedel-Crafts Reaction Alkylation • The introduction of an alkyl group onto the benzene ring • Called the Friedel-Crafts reaction after its discoverers • Among the most useful electrophilic aromatic substitution ...
... Rings: The Friedel-Crafts Reaction Alkylation • The introduction of an alkyl group onto the benzene ring • Called the Friedel-Crafts reaction after its discoverers • Among the most useful electrophilic aromatic substitution ...
Exam 3 - Napa Valley College
... mean that you would get a lot of by-products but you would end up getting more product also (SN1 major, E1 minor). 4) There are a number of ways of substituting a halogen for an alcohol group, but some ways are better than others. What advantage is there in using PCl3 rather than HCl in the chloride ...
... mean that you would get a lot of by-products but you would end up getting more product also (SN1 major, E1 minor). 4) There are a number of ways of substituting a halogen for an alcohol group, but some ways are better than others. What advantage is there in using PCl3 rather than HCl in the chloride ...
Highly Enantioselective Cyclocarbonylation of Allylic
... asymmetric cyclocarbonylation of the six-membered ring allylicalcohol 3e using a Pd-BICP catalyst. At 80 °C, chiral γ-butyrolactone 4e was formed in 93% ee and 87% yield (entry 8). Upon lowering the reaction temperature to 60 °C, up to 98% ee was achieved (entry 9). A derivative (4f) of chiral γ-but ...
... asymmetric cyclocarbonylation of the six-membered ring allylicalcohol 3e using a Pd-BICP catalyst. At 80 °C, chiral γ-butyrolactone 4e was formed in 93% ee and 87% yield (entry 8). Upon lowering the reaction temperature to 60 °C, up to 98% ee was achieved (entry 9). A derivative (4f) of chiral γ-but ...
98 pts
... B. (4 pts) Account for the difference in products in the two reactions (Show explicit structures; I will not accept a verbal only explanation!! More structures, fewer words!). Dehydration: It occurs via E1 mechanism. A carbocation is formed as an intermediate and it generates the alkene products ac ...
... B. (4 pts) Account for the difference in products in the two reactions (Show explicit structures; I will not accept a verbal only explanation!! More structures, fewer words!). Dehydration: It occurs via E1 mechanism. A carbocation is formed as an intermediate and it generates the alkene products ac ...
lec-3- 211( Elim+ Re..
... Asian flush, Asian glow, among others) is a condition in which an individual's face or body experiences flushes or blotches as a result of an accumulation of acetaldehyde, a metabolic byproduct of the catabolic metabolism of alcohol. It is commonly thought that the flush reaction is caused by an ina ...
... Asian flush, Asian glow, among others) is a condition in which an individual's face or body experiences flushes or blotches as a result of an accumulation of acetaldehyde, a metabolic byproduct of the catabolic metabolism of alcohol. It is commonly thought that the flush reaction is caused by an ina ...
Chlorotrimethylsilane/Sodium Iodide, a
... carboxylic acids (4) were isolated in excellent yield (eq 1). The reaction is quite facile for methyl, ethyl, and benzyl esters. Benzyl esters are cleaved a t ambient temperature, whereas methyl and ethyl esters require heating under reflux. Even hindered esters [e.g., methyl pivalate ( l g ) ] can ...
... carboxylic acids (4) were isolated in excellent yield (eq 1). The reaction is quite facile for methyl, ethyl, and benzyl esters. Benzyl esters are cleaved a t ambient temperature, whereas methyl and ethyl esters require heating under reflux. Even hindered esters [e.g., methyl pivalate ( l g ) ] can ...
Vinylcyclopropane rearrangement

The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.Due to its ability to form cyclopentene rings the vinylcyclopropane rearrangement has served several times as a key reaction in complex natural product synthesis.