
Synthetic applications of ortho esters
... ortho esters can been achieved. In addition to asymmetric catalysis, starting materials from the chiral pool can be used for the synthesis of the (R)- or (S)-3-methyl-3,4-epoxy-1-butanol component of the epoxy ester–ortho ester rearrangement. For example, commercially available citramalic acid 24 is ...
... ortho esters can been achieved. In addition to asymmetric catalysis, starting materials from the chiral pool can be used for the synthesis of the (R)- or (S)-3-methyl-3,4-epoxy-1-butanol component of the epoxy ester–ortho ester rearrangement. For example, commercially available citramalic acid 24 is ...
Catalytic Enantioselective Dibromination of Allylic Alcohols
... yields in most cases were moderate, diastereomeric dibromide products were not observed; starting material and malonate− alcohol transesterification products composed the majority of the remaining material. In the case of a p-vinyl substrate (entry 11), dibromination at the terminal olefin was not obs ...
... yields in most cases were moderate, diastereomeric dibromide products were not observed; starting material and malonate− alcohol transesterification products composed the majority of the remaining material. In the case of a p-vinyl substrate (entry 11), dibromination at the terminal olefin was not obs ...
Applications of Phosphorus, Sulfur, Silicon and Boron Chemistry:
... Due to its electron deficiency borane forms the dimer diborane (B2H6). Two-electron three-centre bonds (i.e bridging hydrogen atoms) are used to explain the bonding in this species. Borane is also commercially available in a variety of forms as a 'complex' with an electron pair donator - i.e. a Lewi ...
... Due to its electron deficiency borane forms the dimer diborane (B2H6). Two-electron three-centre bonds (i.e bridging hydrogen atoms) are used to explain the bonding in this species. Borane is also commercially available in a variety of forms as a 'complex' with an electron pair donator - i.e. a Lewi ...
Catalytic asymmetric carbonyl addition reactions catalysed by group
... Catalytic asymmetric carbonyl addition reactions catalysed by group 10 metals The addition of a nucleophilic species to the carbonyl group is one of the most important methodology for carbon-carbon bond construction and various solutions have been offered to achieve an asymmetric version. ...
... Catalytic asymmetric carbonyl addition reactions catalysed by group 10 metals The addition of a nucleophilic species to the carbonyl group is one of the most important methodology for carbon-carbon bond construction and various solutions have been offered to achieve an asymmetric version. ...
- Opus: Online Publications Store
... bond in a molecule it is necessary to activate this bond (making it more reactive) as the first step, followed by its subsequent functionalization [1a]. The activation process can be achieved effectively by using transition metal catalysts and others [1c,11]. It is well−known that complexes based on ...
... bond in a molecule it is necessary to activate this bond (making it more reactive) as the first step, followed by its subsequent functionalization [1a]. The activation process can be achieved effectively by using transition metal catalysts and others [1c,11]. It is well−known that complexes based on ...
HPLC and LC–MS Studies of the Transesterification Reaction of
... positional isomers at the collection stage, the vials were kept on a bed of ice between collections. When the fraction collection was done, the mobile phase in the collected samples was evaporated overnight to dryness with a SpeedVac Plus Concentrator (Model SC210A) (Holbrook, NY). The dry samples w ...
... positional isomers at the collection stage, the vials were kept on a bed of ice between collections. When the fraction collection was done, the mobile phase in the collected samples was evaporated overnight to dryness with a SpeedVac Plus Concentrator (Model SC210A) (Holbrook, NY). The dry samples w ...
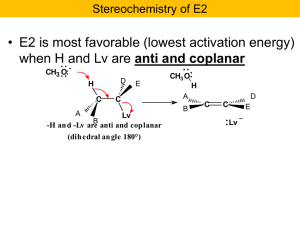
Chapter 8 I. Nucleophilic Substitution
... at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected mechanism. In this new pathway, called the roundabout mechanism, chlor ...
... at the University of Freiburg, in Germany, in collaboration with William L. Hase's group at Texas Tech University, provide direct evidence for this mechanism in the gas phase. However, they also detected an additional, unexpected mechanism. In this new pathway, called the roundabout mechanism, chlor ...
Chapter 24. Amines
... • Primary, secondary, and tertiary amines all have similar reactivity, the initially formed monoalkylated substance undergoes further reaction to yield a mixture of products ...
... • Primary, secondary, and tertiary amines all have similar reactivity, the initially formed monoalkylated substance undergoes further reaction to yield a mixture of products ...
Chapter 24. Amines
... • Primary, secondary, and tertiary amines all have similar reactivity, the initially formed monoalkylated substance undergoes further reaction to yield a mixture of products ...
... • Primary, secondary, and tertiary amines all have similar reactivity, the initially formed monoalkylated substance undergoes further reaction to yield a mixture of products ...
10.3 PREPARATION OF ETHERS
... However, this reaction is much less useful when ammonia is the nucleophile because the initial product, a primary amine, is a stronger base and a stronger nucleophile than is ammonia. Therefore, the primary amine preferentially reacts as the nucleophile, producing a secondary amine as a by-product ( ...
... However, this reaction is much less useful when ammonia is the nucleophile because the initial product, a primary amine, is a stronger base and a stronger nucleophile than is ammonia. Therefore, the primary amine preferentially reacts as the nucleophile, producing a secondary amine as a by-product ( ...
Organic Chemistry, 11th Edition
... Founded in 1807, John Wiley & Sons, Inc. has been a valued source of knowledge and understanding for more than 200 years, helping people around the world meet their needs and fulfill their aspirations. Our company is built on a foundation of principles that include responsibility to the communities ...
... Founded in 1807, John Wiley & Sons, Inc. has been a valued source of knowledge and understanding for more than 200 years, helping people around the world meet their needs and fulfill their aspirations. Our company is built on a foundation of principles that include responsibility to the communities ...
Synthesis of [RuCl2(NO)2(THF)] and its Double CN BondForming
... attractive approach in organic synthesis. It is in this context that we are interested in the ligandbased reaction of metal nitrosyl complexes with alkenes.[5] In the absence of metal coordination the reaction chemistry of NO with alkenes is unruly.[4] Furthermore, only a few transition-metal comple ...
... attractive approach in organic synthesis. It is in this context that we are interested in the ligandbased reaction of metal nitrosyl complexes with alkenes.[5] In the absence of metal coordination the reaction chemistry of NO with alkenes is unruly.[4] Furthermore, only a few transition-metal comple ...
Organic Chemistry
... • it is used in catalytic amounts with another oxidizing agent to reoxidize its reduced forms and, thus, recycle ...
... • it is used in catalytic amounts with another oxidizing agent to reoxidize its reduced forms and, thus, recycle ...
Gas-Phase Reactions of Fe (CH2O)+ and Fe (CH2S)+ with Small
... Formaldehyde was chosen since it is the simplest hydrocarbon containing oxygen, and an understanding of its ligand effects on Fe+ may provide information on the mechanism of hydroformylation and many other catalytic processes, such as those involved in the synthesis of aldehydes and other oxygenated ...
... Formaldehyde was chosen since it is the simplest hydrocarbon containing oxygen, and an understanding of its ligand effects on Fe+ may provide information on the mechanism of hydroformylation and many other catalytic processes, such as those involved in the synthesis of aldehydes and other oxygenated ...
Full Text - Journal of the Indian Institute of Science
... from alcohols. However, it went into oblivion for nearly a decade soon after its discovery by E. M. Burgess in 1968. It was Peter Wipf who brought it to the attention of organic chemists through its extensive use in the formation of 5-membered heterocycles from their acyclic precursors. An interesti ...
... from alcohols. However, it went into oblivion for nearly a decade soon after its discovery by E. M. Burgess in 1968. It was Peter Wipf who brought it to the attention of organic chemists through its extensive use in the formation of 5-membered heterocycles from their acyclic precursors. An interesti ...
Alkyl halide
... • Alkyl halide chemistry acts as a relatively simple model for many mechanistically similar but structurally more complex reactions found in biomolecules ...
... • Alkyl halide chemistry acts as a relatively simple model for many mechanistically similar but structurally more complex reactions found in biomolecules ...
Ethers and Epoxides
... Ring-Opening Reactions of Epoxides • Water adds to epoxides with dilute acid at room temperature • Product is a 1,2-diol (on adjacent C’s: vicinal) • Mechanism: acid protonates oxygen and water adds to opposite side (trans addition) ...
... Ring-Opening Reactions of Epoxides • Water adds to epoxides with dilute acid at room temperature • Product is a 1,2-diol (on adjacent C’s: vicinal) • Mechanism: acid protonates oxygen and water adds to opposite side (trans addition) ...
Rapid Ether and Alcohol CO Bond Hydrogenolysis Catalyzed by
... broad spectrum of biomass-related substrates. This approach is advantageous over previous protocols from this laboratory12,14 and others in many ways: (1) extended substrate scope including C−O bonds of alcohols and primary ethers; (2) high yields of alkanes without skeletal rearrangement; (3) comme ...
... broad spectrum of biomass-related substrates. This approach is advantageous over previous protocols from this laboratory12,14 and others in many ways: (1) extended substrate scope including C−O bonds of alcohols and primary ethers; (2) high yields of alkanes without skeletal rearrangement; (3) comme ...
Phosphine-Catalyzed Additions of Nucleophiles and Electrophiles to
... and electrophiles with α,β-unsaturated carbonyl compounds. Although some of the reactions are limited by substrate specificity and long reaction times, this class of reactions has become a useful tool in organic synthesis. This is illustrated by applications in the production of polymers25 and the s ...
... and electrophiles with α,β-unsaturated carbonyl compounds. Although some of the reactions are limited by substrate specificity and long reaction times, this class of reactions has become a useful tool in organic synthesis. This is illustrated by applications in the production of polymers25 and the s ...
Chapter 22: Phenols. Alcohols contain an OH group bonded to an
... OH group of phenols can participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
... OH group of phenols can participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols pKa ~ 16 H3CH2C O H ...
List of Objectives for Chem52
... *Draw the products and mechanisms (including all electron-pushing arrows, intermediates, and by-products) of nucleophilic aromatic substitution reactions, both through the elimination/ addition mechanism through benzyne and the addition/elimination mechanism (SNAr). Prepare benzyne intermediates thr ...
... *Draw the products and mechanisms (including all electron-pushing arrows, intermediates, and by-products) of nucleophilic aromatic substitution reactions, both through the elimination/ addition mechanism through benzyne and the addition/elimination mechanism (SNAr). Prepare benzyne intermediates thr ...
Copper(II) bromide as efficient catalyst for silyl
... The bis(methoxyphenyl)methyl (BMPM) ether derived from hexanol was produced in every case investigated, but at different rates. Monitoring the reaction revealed that TES, TPS, and TBS ethers exhibited a higher kinetic interconversion at the beginning of the transformation compared to TIPS and TBDPS ...
... The bis(methoxyphenyl)methyl (BMPM) ether derived from hexanol was produced in every case investigated, but at different rates. Monitoring the reaction revealed that TES, TPS, and TBS ethers exhibited a higher kinetic interconversion at the beginning of the transformation compared to TIPS and TBDPS ...
Nonracemic Allylic Boronates through Enantiotopic-Group
... amines by oxidation and amination, but they can also engage in carbonyl allylations that establish chiral all-carbon quaternary centers.1 However, in spite of their ability to address important synthesis problems, there remain few catalytic enantioselective methods for the construction of α-chiral a ...
... amines by oxidation and amination, but they can also engage in carbonyl allylations that establish chiral all-carbon quaternary centers.1 However, in spite of their ability to address important synthesis problems, there remain few catalytic enantioselective methods for the construction of α-chiral a ...
CHEM120 - ORGANIC CHEMISTRY WORKSHEET 1
... You must know what general elimination reactions and what SN1 and SN2 reactions stand for. How many molecules are involved in the ratedetermining step of each of these SN1 and SN2 reactions. ...
... You must know what general elimination reactions and what SN1 and SN2 reactions stand for. How many molecules are involved in the ratedetermining step of each of these SN1 and SN2 reactions. ...
Vinylcyclopropane rearrangement

The vinylcyclopropane rearrangement or vinylcyclopropane-cyclopentene rearrangement is a ring expansion reaction, converting a vinyl-substituted cyclopropane ring into a cyclopentene ring.Intense experimental as well as computational investigations have revealed that mechanistically, the vinylcyclopropane rearrangement can be thought of as either a diradical-mediated two-step and/or orbital-symmetry-controlled pericyclic process. The amount by which each of the two mechanisms is operative is highly dependent on the substrate.Due to its ability to form cyclopentene rings the vinylcyclopropane rearrangement has served several times as a key reaction in complex natural product synthesis.









![Synthesis of [RuCl2(NO)2(THF)] and its Double CN BondForming](http://s1.studyres.com/store/data/001773792_1-763ad0089529123821e01ed17077bbf2-300x300.png)











