Chapter 8. CARBONYL COMPOUNDS
... Aldehydes and ketones possess very weak acidity and basicity, therefore they cannot form intermolecular hydrogen bonds. Carbonyl compounds are more volatile than the corresponding alcohols. Compare, for example, boiling points of propanal (49 °C), acetone (56 °C), and 1propanol (97 °C). 8.2. NUCLEOP ...
... Aldehydes and ketones possess very weak acidity and basicity, therefore they cannot form intermolecular hydrogen bonds. Carbonyl compounds are more volatile than the corresponding alcohols. Compare, for example, boiling points of propanal (49 °C), acetone (56 °C), and 1propanol (97 °C). 8.2. NUCLEOP ...
Reactions of Alcohols
... -OH, to promote the reaction. • The chloride product is insoluble. • Lucas test: ZnCl2 in conc. HCl 1° alcohols react slowly or not at all. 2 alcohols react in 1-5 minutes. 3 alcohols react in less than 1 minute. ...
... -OH, to promote the reaction. • The chloride product is insoluble. • Lucas test: ZnCl2 in conc. HCl 1° alcohols react slowly or not at all. 2 alcohols react in 1-5 minutes. 3 alcohols react in less than 1 minute. ...
Types of reactions you know:
... It’s sometimes helpful to group reactions according to what they are useful for in synthesis: 1) Adding alkyl groups – if the product has more carbons than the starting material. a. Add a primary alkyl halide to a terminal alkyne (an SN2 rxn) ...
... It’s sometimes helpful to group reactions according to what they are useful for in synthesis: 1) Adding alkyl groups – if the product has more carbons than the starting material. a. Add a primary alkyl halide to a terminal alkyne (an SN2 rxn) ...
Elimination Reactions
... have higher entropies than substitutions because eliminations have a greater number of products formed than that of starting compounds). • Any substitution that occurs must take place through an SN1 mechanism ...
... have higher entropies than substitutions because eliminations have a greater number of products formed than that of starting compounds). • Any substitution that occurs must take place through an SN1 mechanism ...
Chapter 8 Alkenes and Alkynes II
... Modern Statement of Markovnikov’s Rule: In the ionic addition of an unsymmetrical reagent to a double bond, the positive portion of the adding reagent attaches itself to a carbon atom of the double bond so as to yield the more stable carbocation as an intermediate ...
... Modern Statement of Markovnikov’s Rule: In the ionic addition of an unsymmetrical reagent to a double bond, the positive portion of the adding reagent attaches itself to a carbon atom of the double bond so as to yield the more stable carbocation as an intermediate ...
File - Dr KHALID SHADID
... Reactions of Carboxylic Acid Anhydrides Because carboxylic acid anhydrides are highly reactive they can be used to prepare esters and amides. ...
... Reactions of Carboxylic Acid Anhydrides Because carboxylic acid anhydrides are highly reactive they can be used to prepare esters and amides. ...
HONORS ORGANIC CHEM. HAHS MRS. RICHARDS 1 ORGANIC
... 1.) Naming: alkenes, alkynes, enynes, alcohols 2.) Reactions: alkenes and alkynes: determining products, reagents, reactants 3.) Mechanisms: alkenes and alkynes: electrophilic addition of HX (both), halohydrin formation (alkenes only), acid-catalyzed hydration (both), hydroboration (both), acetylide ...
... 1.) Naming: alkenes, alkynes, enynes, alcohols 2.) Reactions: alkenes and alkynes: determining products, reagents, reactants 3.) Mechanisms: alkenes and alkynes: electrophilic addition of HX (both), halohydrin formation (alkenes only), acid-catalyzed hydration (both), hydroboration (both), acetylide ...
Chapter 18
... Hydrolysis can occur under either acidic or basic mechanism, although reaction under basic conditions can lead to other types of reactions so acidic hydrolysis is preferred ...
... Hydrolysis can occur under either acidic or basic mechanism, although reaction under basic conditions can lead to other types of reactions so acidic hydrolysis is preferred ...
protecting groups
... For aldehydes and ketones, protetion usually involves the formation of an acetal or ketal, the five- and six-membered cyclic derivatives (1, 3-dioxolanes and 1,3-dioxanes, respectively) being particularly important. Deprotection involves acid hydrolysys. The formation of these cyclic acetals and ket ...
... For aldehydes and ketones, protetion usually involves the formation of an acetal or ketal, the five- and six-membered cyclic derivatives (1, 3-dioxolanes and 1,3-dioxanes, respectively) being particularly important. Deprotection involves acid hydrolysys. The formation of these cyclic acetals and ket ...
Physical Organic Chemistry
... without substituted with another atom or group. The elimination of HX molecule from alkyl derivatives. While X is a halogen or ester… etc. the hydrogen atom on adjacent carbon with X Elimination reactions and nucleophilic substitution are similar in cases of affecting factors. Hence it’s a com ...
... without substituted with another atom or group. The elimination of HX molecule from alkyl derivatives. While X is a halogen or ester… etc. the hydrogen atom on adjacent carbon with X Elimination reactions and nucleophilic substitution are similar in cases of affecting factors. Hence it’s a com ...
Exam 3 Key - Chemistry
... b) initial attack by Hc) initial loss of LiH d) loss of Al e) loss of water 2. (3) Reduction by BH3 always involves: a) initial protonation by H+ b) initial attack by Hc) loss of H2 d) initial attack at the boron e) addition of water 3. (3) By analogy with what you have seen before, the reaction of ...
... b) initial attack by Hc) initial loss of LiH d) loss of Al e) loss of water 2. (3) Reduction by BH3 always involves: a) initial protonation by H+ b) initial attack by Hc) loss of H2 d) initial attack at the boron e) addition of water 3. (3) By analogy with what you have seen before, the reaction of ...
Alkenes Key features sp -hybridized carbons, 120 bond angles
... most stable carbocation intermediate Markovnikov's Rule Restated: "The rich get richer rule" For electrophilic addition to alkenes, the hydrogen goes to the carbon atom which is already bonded to the greatest number of hydrogens (also results in more stable carbocation structure) ...
... most stable carbocation intermediate Markovnikov's Rule Restated: "The rich get richer rule" For electrophilic addition to alkenes, the hydrogen goes to the carbon atom which is already bonded to the greatest number of hydrogens (also results in more stable carbocation structure) ...
ch18 by dr. dina
... Acid Chlorides Synthesis of Acid Chlorides Acid chlorides are made from carboxylic acids by reaction with thionyl chloride, phosphorus trichloride or phosphorus pentachloride These reagents work because they turn the hydroxyl group of the carboxylic acid into an excellent leaving group ...
... Acid Chlorides Synthesis of Acid Chlorides Acid chlorides are made from carboxylic acids by reaction with thionyl chloride, phosphorus trichloride or phosphorus pentachloride These reagents work because they turn the hydroxyl group of the carboxylic acid into an excellent leaving group ...
- Iranian Journal of Science and Technology (Sciences)
... obtained from the reaction of triethylamine and chlorodiphenylphosphine was selected as the most suitable reagent and media for performing the esterification reactions. Apart from the ease of handling and preparation of this IL, the phosphine oxide formed in the reaction is taken up by the ionic liq ...
... obtained from the reaction of triethylamine and chlorodiphenylphosphine was selected as the most suitable reagent and media for performing the esterification reactions. Apart from the ease of handling and preparation of this IL, the phosphine oxide formed in the reaction is taken up by the ionic liq ...
Document
... eliminations. Iodine has the weakest bond to carbon, and iodide is the best leaving group. Alkyl iodides are several times more reactive than alkyl bromides and from 50 to 100 times more reactive than alkyl chlorides. Fluorine has the strongest bond to carbon, and fluoride is the poorest leaving gro ...
... eliminations. Iodine has the weakest bond to carbon, and iodide is the best leaving group. Alkyl iodides are several times more reactive than alkyl bromides and from 50 to 100 times more reactive than alkyl chlorides. Fluorine has the strongest bond to carbon, and fluoride is the poorest leaving gro ...
Lab 9 - Academic Computer Center
... The overall reduction of a carbonyl group to a hydroxyl group involves the addition of two H atoms. The first H atom comes from a hydride, H-, of NaBH4. The second comes from the workup of the reaction, which is normally conducted in aqueous acid. Sodium borohydride, NaBH4, is the mildest of the th ...
... The overall reduction of a carbonyl group to a hydroxyl group involves the addition of two H atoms. The first H atom comes from a hydride, H-, of NaBH4. The second comes from the workup of the reaction, which is normally conducted in aqueous acid. Sodium borohydride, NaBH4, is the mildest of the th ...
Carboxylic Acid Derivatives and Nucleophilic Acyl Substitution
... substitution of a carboxylic acid When 18O-labeled methanol reacts with benzoic acid, the methyl benzoate produced is 18O-labeled but the water produced is unlabeled ...
... substitution of a carboxylic acid When 18O-labeled methanol reacts with benzoic acid, the methyl benzoate produced is 18O-labeled but the water produced is unlabeled ...
CARBONYL COMPOUNDS - Aldehydes and Ketones C=O C C C
... • contains copper(II) ions complexed with tartrate ions giving a blue solution • on warming, it will oxidise aliphatic (but not aromatic) aldehydes • copper(II) is reduced and a red precipitate of copper(I) oxide, Cu2O, is formed The silver mirror test is the better alternative as it works with all ...
... • contains copper(II) ions complexed with tartrate ions giving a blue solution • on warming, it will oxidise aliphatic (but not aromatic) aldehydes • copper(II) is reduced and a red precipitate of copper(I) oxide, Cu2O, is formed The silver mirror test is the better alternative as it works with all ...
reductive elimination
... Rate of RE lower for B than A because cis to trans isomerization must occur before RE can occur. ...
... Rate of RE lower for B than A because cis to trans isomerization must occur before RE can occur. ...
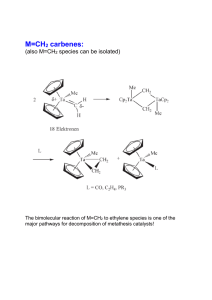
Steric protection of alkylidene is not needed:
... The bimolecular reaction of M=CH2 to ethylene species is one of the major pathways for decomposition of metathesis catalysts! ...
... The bimolecular reaction of M=CH2 to ethylene species is one of the major pathways for decomposition of metathesis catalysts! ...
Alcohols
... Ethanol is the least toxic alcohol, but it is still toxic. The body detoxifies ethanol with NAD catalyzed first by alcohol dehydrogenase (ADH) and second by aldehyde dehydrogenase (ALDH): ethanol acetic acid The reason methanol and ethylene glycol are so toxic to humans is that, when they ...
... Ethanol is the least toxic alcohol, but it is still toxic. The body detoxifies ethanol with NAD catalyzed first by alcohol dehydrogenase (ADH) and second by aldehyde dehydrogenase (ALDH): ethanol acetic acid The reason methanol and ethylene glycol are so toxic to humans is that, when they ...
22.4: Acidity of Phenols.
... 22.3: Physical Properties (please read). Like other alcohols the OH group of phenols cab participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols ...
... 22.3: Physical Properties (please read). Like other alcohols the OH group of phenols cab participate in hydrogen bonding with other phenol molecules and to water. 22.4: Acidity of Phenols. Phenols are more acidic than aliphatic alcohols ...
19.2 preparation of acyl chlorides
... Amides are less reactive than esters, and their hydrolysis often requires vigorous heating in either aqueous acid or base. The mechanism for acidic conditions is quite similar to the reverse of the Fischer esterification mechanism shown in Figure 19.3. The mechanism for basic conditions is related t ...
... Amides are less reactive than esters, and their hydrolysis often requires vigorous heating in either aqueous acid or base. The mechanism for acidic conditions is quite similar to the reverse of the Fischer esterification mechanism shown in Figure 19.3. The mechanism for basic conditions is related t ...
Wolff rearrangement

The Wolff rearrangement is a reaction in organic chemistry in which an α-diazocarbonyl compound is converted into a ketene by loss of dinitrogen with accompanying 1,2-rearrangement. The Wolff rearrangement yields a ketene as an intermediate product, which can undergo nucleophilic attack with weakly acidic nucleophiles such as water, alcohols, and amines, to generate carboxylic acid derivatives or undergo [2+2] cycloaddition reactions to form four-membered rings. The mechanism of the Wolff rearrangement has been the subject of debate since its first use. No single mechanism sufficiently describes the reaction, and there are often competing concerted and carbene-mediated pathways; for simplicity, only the textbook, concerted mechanism is shown below. The reaction was discovered by Ludwig Wolff in 1902. The Wolff rearrangement has great synthetic utility due to the accessibility of α-diazocarbonyl compounds, variety of reactions from the ketene intermediate, and stereochemical retention of the migrating group. However, the Wolff rearrangement has limitations due to the highly reactive nature of α-diazocarbonyl compounds, which can undergo a variety of competing reactions.The Wolff rearrangement can be induced via thermolysis, photolysis, or transition metal catalysis. In this last case, the reaction is sensitive to the transition metal; silver (I) oxide or other Ag(I) catalysts work well and are generally used. The Wolff rearrangement has been used in many total syntheses; the most common use is trapping the ketene intermediate with nucleophiles to form carboxylic acid derivatives. The Arndt-Eistert homologation is a specific example of this use, wherein a carboxylic acid may be elongated by a methylene unit. Another common use is in ring-contraction methods; if the α-diazo ketone is cyclic, the Wolff rearrangement results in a ring-contracted product. The Wolff rearrangement works well in generating ring-strained systems, where other reactions may fail.