Scientific Method - Virtual Medical Academy
... Number of Electrons:>>> * An atom is neutral. * The net charge is zero. * Number of protons = Number of electrons. * Atomic number = Number of electrons. * Mass number = Number of protons + Number of neutrons. Examples>> ...
... Number of Electrons:>>> * An atom is neutral. * The net charge is zero. * Number of protons = Number of electrons. * Atomic number = Number of electrons. * Mass number = Number of protons + Number of neutrons. Examples>> ...
General Chemistry Chapter 3 Note Packet
... atomic mass = #p+ + #n0 The total charge on an atom is determined by the number of p+ and e-. Since these particles have charges of _________ magnitude and _____________ sign, their charges cancel. When an atom has the same number of p+ and e- , the total charge must be zero – a neutral atom. • An I ...
... atomic mass = #p+ + #n0 The total charge on an atom is determined by the number of p+ and e-. Since these particles have charges of _________ magnitude and _____________ sign, their charges cancel. When an atom has the same number of p+ and e- , the total charge must be zero – a neutral atom. • An I ...
IT IS ELEMENTARY - the OLLI at UCI Blog
... AND FINALLY • Structure of an atom: nucleus and electrons • Nucleus: consists of protons and neutrons • Atomic number: number of protons in the nucleus of an atom • Atomic mass number: number of protons plus neutrons in the nucleus of an atom • Isotopes: atoms with the same atomic number and a diff ...
... AND FINALLY • Structure of an atom: nucleus and electrons • Nucleus: consists of protons and neutrons • Atomic number: number of protons in the nucleus of an atom • Atomic mass number: number of protons plus neutrons in the nucleus of an atom • Isotopes: atoms with the same atomic number and a diff ...
Constructing an Atom We`re going sub-atomic!
... Valence Electrons • Valence electrons- these are the electrons on the outer most orbit of the atom. – This does not include all the electrons just the electrons on the outer orbit of the atom. – Valence Electrons are responsible for atoms to react to each other!!! ...
... Valence Electrons • Valence electrons- these are the electrons on the outer most orbit of the atom. – This does not include all the electrons just the electrons on the outer orbit of the atom. – Valence Electrons are responsible for atoms to react to each other!!! ...
Structure of the Atom: Study Guide
... b. object B, density is 1.95 g/ml c. object C, density is 0.83 g/ml d. none of the above SHORT ANSWERS 1) What is the smallest unit of matter that retains the identity of the substance? 2) Atoms are composed of two regions, what are they and what do they contain? 3) What does the Atomic Number tell ...
... b. object B, density is 1.95 g/ml c. object C, density is 0.83 g/ml d. none of the above SHORT ANSWERS 1) What is the smallest unit of matter that retains the identity of the substance? 2) Atoms are composed of two regions, what are they and what do they contain? 3) What does the Atomic Number tell ...
Why are atoms of lead different to those of gold and why can we not
... Activity 5 Atoms contain three different particles Electrons are negatively charged particles that orbit a mass at the centre of the atom. The centre of the atom is known as the nucleus. This contains positive proton particles and neutral neutron particles. ...
... Activity 5 Atoms contain three different particles Electrons are negatively charged particles that orbit a mass at the centre of the atom. The centre of the atom is known as the nucleus. This contains positive proton particles and neutral neutron particles. ...
Unit 3 – Atomic Theory
... If Thompson’s model were true, the “shadow” would appear as a somewhat random distribution, as the protons should have no ...
... If Thompson’s model were true, the “shadow” would appear as a somewhat random distribution, as the protons should have no ...
atom
... be. Atoms with electrons in higher energy levels have additional electron clouds of different shapes that also show where those electrons are likely to be. For more information, click here: http://regentsprep.org/Regents/physics/phys05/catomodel/cloud.htm ...
... be. Atoms with electrons in higher energy levels have additional electron clouds of different shapes that also show where those electrons are likely to be. For more information, click here: http://regentsprep.org/Regents/physics/phys05/catomodel/cloud.htm ...
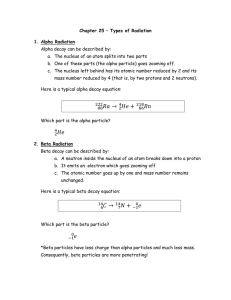
Chapter 25 – Types of Radiation 1. Alpha Radiation Alpha decay
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
... Positron decay is the mirror image of beta decay and can be described as: a. Something inside the nucleus breaks down causing a proton to become a neutron. b. It emits a positron which goes zooming off. c. The atomic number goes down by one and the mass number remains unchanged. Here is a typical po ...
Atoms
... As early as 400 BC scientists have believed in an atomic theory thanks to Democritus. n Atoms were the building blocks of matter. n 2000 years later we can see the atom! n ...
... As early as 400 BC scientists have believed in an atomic theory thanks to Democritus. n Atoms were the building blocks of matter. n 2000 years later we can see the atom! n ...
Which of the following statements correctly describes the
... Which of the following statements accurately describes the locations of the three subatomic particles that make up an atom? A ...
... Which of the following statements accurately describes the locations of the three subatomic particles that make up an atom? A ...
Lesson Overview
... The total number of protons and neutrons in the nucleus of an atom is called its mass number. Isotopes are identified by their mass numbers; for example, carbon-12, carbon-13, and carbon-14. The weighted average of the masses of an element’s isotopes, in which the abundance of each isotope in nature ...
... The total number of protons and neutrons in the nucleus of an atom is called its mass number. Isotopes are identified by their mass numbers; for example, carbon-12, carbon-13, and carbon-14. The weighted average of the masses of an element’s isotopes, in which the abundance of each isotope in nature ...
Atomic structure and radioactive decay
... Gamma decay Gamma radiation is a form of electromagnetic radiation, not a type of particle. When an atom’s nucleus decays and emits gamma radiation, there is no change to the make-up of the nucleus and so a new element is not formed. Gamma rays are usually emitted with alpha or beta particles. For ...
... Gamma decay Gamma radiation is a form of electromagnetic radiation, not a type of particle. When an atom’s nucleus decays and emits gamma radiation, there is no change to the make-up of the nucleus and so a new element is not formed. Gamma rays are usually emitted with alpha or beta particles. For ...
Chemistry Notes: Chapter 1.1
... different type of atom. There are over 100 known naturally occurring elements. The smallest particle that makes up any type of element. All matter is made of atoms. Atoms are very very small. An atom is made up of 3 charged particles: 1. Protons—have a positive (+) charge 2. Neutrons—have no (o) cha ...
... different type of atom. There are over 100 known naturally occurring elements. The smallest particle that makes up any type of element. All matter is made of atoms. Atoms are very very small. An atom is made up of 3 charged particles: 1. Protons—have a positive (+) charge 2. Neutrons—have no (o) cha ...
7th Science - Carterville CUSD #5
... 12. If you were given a periodic table, how could you figure out how many protons an atom of Nickel has? ...
... 12. If you were given a periodic table, how could you figure out how many protons an atom of Nickel has? ...
Document
... In figure 26, the blocks for most of the elements are _________________________. These elements are __________. The blocks for ______________ are ____________. Between the metals and the nonmetals are the _________________. These elements are represented by the _______________________________. Semim ...
... In figure 26, the blocks for most of the elements are _________________________. These elements are __________. The blocks for ______________ are ____________. Between the metals and the nonmetals are the _________________. These elements are represented by the _______________________________. Semim ...
Isotope

Isotopes are variants of a particular chemical element which differ in neutron number, although all isotopes of a given element have the same number of protons in each atom. The term isotope is formed from the Greek roots isos (ἴσος ""equal"") and topos (τόπος ""place""), meaning ""the same place""; thus, the meaning behind the name it is that different isotopes of a single element occupy the same position on the periodic table. The number of protons within the atom's nucleus is called atomic number and is equal to the number of electrons in the neutral (non-ionized) atom. Each atomic number identifies a specific element, but not the isotope; an atom of a given element may have a wide range in its number of neutrons. The number of nucleons (both protons and neutrons) in the nucleus is the atom's mass number, and each isotope of a given element has a different mass number.For example, carbon-12, carbon-13 and carbon-14 are three isotopes of the element carbon with mass numbers 12, 13 and 14 respectively. The atomic number of carbon is 6, which means that every carbon atom has 6 protons, so that the neutron numbers of these isotopes are 6, 7 and 8 respectively.