Production of Materials by Jimmy Huang
... The alkene further splits into smaller alkenes until either ethylene or propene (or both) is formed e.g. C5H10 C2H4 + C3H6. Therefore, the overall products of catalytic cracking are alkanes of shorter chain lengths and small alkenes. The catalysts used are inorganic compounds known as zeolites, wh ...
... The alkene further splits into smaller alkenes until either ethylene or propene (or both) is formed e.g. C5H10 C2H4 + C3H6. Therefore, the overall products of catalytic cracking are alkanes of shorter chain lengths and small alkenes. The catalysts used are inorganic compounds known as zeolites, wh ...
chemical*equations
... “Success'is'not',inal,'failure' is'not'fatal:'it'is'the'courage' to'continue'that'counts.” ''7Winston'Churchill ...
... “Success'is'not',inal,'failure' is'not'fatal:'it'is'the'courage' to'continue'that'counts.” ''7Winston'Churchill ...
Atomic Energy for Military Purposes
... 1.18. In the decade following Rutherford's work many similar experiments were performed with similar results. One series of experiments of this type led to the discovery of the neutron, which will be discussed in some detail since the neutron is practically the theme song of this whole project. 1.19 ...
... 1.18. In the decade following Rutherford's work many similar experiments were performed with similar results. One series of experiments of this type led to the discovery of the neutron, which will be discussed in some detail since the neutron is practically the theme song of this whole project. 1.19 ...
Introduction to Chemistry
... Ionic- Two elements bond by transferring electrons to create ions that attract together (+ is attracted to - after an electron is transferred) ...
... Ionic- Two elements bond by transferring electrons to create ions that attract together (+ is attracted to - after an electron is transferred) ...
Chemistry 199 - Oregon State chemistry
... to solve we need to take the antilog (ex)of both sides: e(ln[At/1000]) = e(-0.6931) At/1000 = 0.5000 At = 500 ...
... to solve we need to take the antilog (ex)of both sides: e(ln[At/1000]) = e(-0.6931) At/1000 = 0.5000 At = 500 ...
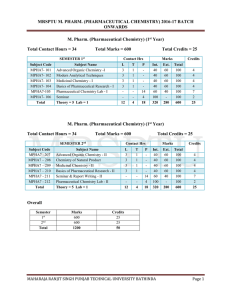
mrsptu m. pharm. (pharmaceutical chemistry) 2016
... 1. Indian Pharmacopoeia, Central Indian Pharmacopoeia Laboratory, Govt. of India, Ministry of Health & Family Welfare, Ghaziabad. 2. U.S. Pharmacopoeia – NF, The United States Pharmacopoeial Convention, Rockville, USA. 3. European Pharmacopoeia, Directorate for the Quality of Medicines of the Counci ...
... 1. Indian Pharmacopoeia, Central Indian Pharmacopoeia Laboratory, Govt. of India, Ministry of Health & Family Welfare, Ghaziabad. 2. U.S. Pharmacopoeia – NF, The United States Pharmacopoeial Convention, Rockville, USA. 3. European Pharmacopoeia, Directorate for the Quality of Medicines of the Counci ...
AP Chemistry - Chagrin Falls Schools
... **If you are at school during any part of the day that an assignment is due, are on a school field trip, or on a planned absence you are required to hand in the assignment to your teacher on the assigned date. Failure to do so will result in an enforcement of the aforementioned late policies. Also, ...
... **If you are at school during any part of the day that an assignment is due, are on a school field trip, or on a planned absence you are required to hand in the assignment to your teacher on the assigned date. Failure to do so will result in an enforcement of the aforementioned late policies. Also, ...
Wales Research and Diagnostic Positron Emission Tomography
... Regulation 2 of the Medicines (Administration of Radioactive Substances) Regulations 1978 (MARS Regulations 1978) requires that any doctor or dentist who wishes to administer radioactive medicinal products to humans should hold a certificate issued by Health Ministers University Post-Graduate Qualif ...
... Regulation 2 of the Medicines (Administration of Radioactive Substances) Regulations 1978 (MARS Regulations 1978) requires that any doctor or dentist who wishes to administer radioactive medicinal products to humans should hold a certificate issued by Health Ministers University Post-Graduate Qualif ...
Physical and Chemical Properties
... • Dmitri Mendeleev, a Russian scientist discovered a set of patterns that seemed to apply to all elements • arranged the elements in order of increasing atomic mass (protons + neutrons in the nucleus) ...
... • Dmitri Mendeleev, a Russian scientist discovered a set of patterns that seemed to apply to all elements • arranged the elements in order of increasing atomic mass (protons + neutrons in the nucleus) ...
Semester 1 Final Review Powerpoint
... molecule because it contains one carbon atom and two oxygen atoms (both are non-metals). ...
... molecule because it contains one carbon atom and two oxygen atoms (both are non-metals). ...
AP Chemistry Summer Assignment
... For those students who have just taken Chemistry 1, much of the material in the summer packet will be familiar to you. For those students who have not taken Chemistry for a while the problems will help you rebuild a foundation in chemistry and insure all students are on a relatively even plane. It w ...
... For those students who have just taken Chemistry 1, much of the material in the summer packet will be familiar to you. For those students who have not taken Chemistry for a while the problems will help you rebuild a foundation in chemistry and insure all students are on a relatively even plane. It w ...
Terminology 1
... (The chemical identity of an atom can be determined solely by it’s atomic number) When the atom is neutral, i.e. not electrically charged, the atomic number equals the number of electrons in its shells ...
... (The chemical identity of an atom can be determined solely by it’s atomic number) When the atom is neutral, i.e. not electrically charged, the atomic number equals the number of electrons in its shells ...
Outline Chapter 10 The Periodic Law
... atom than to the other, making one part of the molecule relatively negative and another part relatively positive. 10-13. Ionic Bond Ionic bond = formed when electrons are transferred between two or more atoms and the resulting ions of opposite charge attract each other. 10-14. Ionic Compounds When a ...
... atom than to the other, making one part of the molecule relatively negative and another part relatively positive. 10-13. Ionic Bond Ionic bond = formed when electrons are transferred between two or more atoms and the resulting ions of opposite charge attract each other. 10-14. Ionic Compounds When a ...