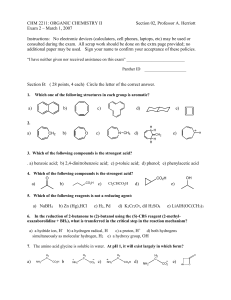
Sample % Sulfate Absolute Deviation A 44.02 B 44.11 C 43.98 D
... 7. Imagine that you briefly heat 84.0 g of a red powder known to be mercuric oxide. After the sample has cooled, you notice a few globs of liquid mercury are mixed together with unreacted red powder. If the mass of the resulting mixture is 82.5 g, how much oxygen was produced during the heating? Whi ...
... 7. Imagine that you briefly heat 84.0 g of a red powder known to be mercuric oxide. After the sample has cooled, you notice a few globs of liquid mercury are mixed together with unreacted red powder. If the mass of the resulting mixture is 82.5 g, how much oxygen was produced during the heating? Whi ...
AP Chemistry Summer Assignment
... 63. A 2.0g sample of SX6 (g) has a volume of 329.5 cm3 at 1.00 atm and 20oC. Identify the element ‘X’. Name the compound. 64. When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a. Write the balanced chemical equation for this reaction. b. How many liters o ...
... 63. A 2.0g sample of SX6 (g) has a volume of 329.5 cm3 at 1.00 atm and 20oC. Identify the element ‘X’. Name the compound. 64. When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a. Write the balanced chemical equation for this reaction. b. How many liters o ...
EVANS GROUP RESEARCH PROJECT DESCRIPTIONS
... chemistry. These include reductive chemistry governed by steric factors rather than the usual electronic effects, reduction with metals in low oxidation states that were thought for decades to be too unstable to exist. This reductive chemistry is utilized in several ways: new methods for reducing di ...
... chemistry. These include reductive chemistry governed by steric factors rather than the usual electronic effects, reduction with metals in low oxidation states that were thought for decades to be too unstable to exist. This reductive chemistry is utilized in several ways: new methods for reducing di ...
CfE Higher Chemistry Homework 3.5
... To avoid these contaminants, hydrogen sulfide can be made by reacting aluminium sulfide with water. Hydrogen sulfide and aluminium hydroxide are produced. Write a balanced chemical equation for the production of hydrogen sulfide from aluminium sulfide and water. ...
... To avoid these contaminants, hydrogen sulfide can be made by reacting aluminium sulfide with water. Hydrogen sulfide and aluminium hydroxide are produced. Write a balanced chemical equation for the production of hydrogen sulfide from aluminium sulfide and water. ...
Chapter 1 Chemistry: Matter and Measurement
... Chemistry is concerned with matter and energy and how the two interact with each other. ...
... Chemistry is concerned with matter and energy and how the two interact with each other. ...
effective nuclear charge
... for transition metals electrons, may be removed from the sublevel closest to the valence shell Al atom = 1s22s22p63s23p1 Al+3 ion = 1s22s22p6 Fe atom = 1s22s22p63s23p64s23d6 Fe+2 ion = 1s22s22p63s23p63d6 Fe+3 ion = 1s22s22p63s23p63d5 Cu atom = 1s22s22p63s23p64s13d10 Cu+1 ion = 1s22s22p63s23p63d10 ...
... for transition metals electrons, may be removed from the sublevel closest to the valence shell Al atom = 1s22s22p63s23p1 Al+3 ion = 1s22s22p6 Fe atom = 1s22s22p63s23p64s23d6 Fe+2 ion = 1s22s22p63s23p63d6 Fe+3 ion = 1s22s22p63s23p63d5 Cu atom = 1s22s22p63s23p64s13d10 Cu+1 ion = 1s22s22p63s23p63d10 ...
Chapter 2: Chemical Basis of Life
... of either the components or the processes of living things without using the biochemist's terms. For example, 96% of the human body is made up of just four major elements. Chemical reactions that hold atoms together do so by forming chemical bonds—these include ionic (electrovalent) bonds, covalent ...
... of either the components or the processes of living things without using the biochemist's terms. For example, 96% of the human body is made up of just four major elements. Chemical reactions that hold atoms together do so by forming chemical bonds—these include ionic (electrovalent) bonds, covalent ...
g - Santa Rosa Junior College
... – The inorganic cycle involves slow weathering of phosphatecontaining rocks, which causes PO43- to leach into the rivers and seas. – The land-based biological cycle involves incorporation of PO43- into organisms and its release through excretion and ...
... – The inorganic cycle involves slow weathering of phosphatecontaining rocks, which causes PO43- to leach into the rivers and seas. – The land-based biological cycle involves incorporation of PO43- into organisms and its release through excretion and ...