AP Chemistry Summer Assignment
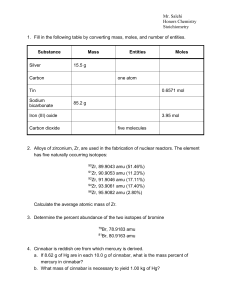
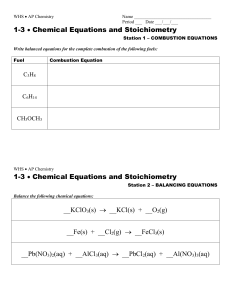
... 60.A 2.0g sample of SX6 (g) has a volume of 329.5 cm3 at 1.00 atm and 20oC. Identify the element ‘X’. Name the compound. 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of su ...
... 60.A 2.0g sample of SX6 (g) has a volume of 329.5 cm3 at 1.00 atm and 20oC. Identify the element ‘X’. Name the compound. 61.When Hydrogen sulfide gas, H2S, reacts with oxygen, Sulfur dioxide gas and steam are produced. a.Write the balanced chemical equation for this reaction. b.How many liters of su ...
chapt 1 - Cantt Academy, Tahli Mohri Chowk, Rawalpindi
... Pseudo – Science or Alchemy:In the beginning of chemistry during 600 – 1600 AD some scientist tried to convert cheap metals in to gold. They performed many experiment but could not succeed and wasted their time and money. These scientists are called alchemists and this branch of chemistry is called ...
... Pseudo – Science or Alchemy:In the beginning of chemistry during 600 – 1600 AD some scientist tried to convert cheap metals in to gold. They performed many experiment but could not succeed and wasted their time and money. These scientists are called alchemists and this branch of chemistry is called ...
Chemistry – V – BSC – 503
... Lectures will be conducted with the aid of multi-media projector, black board, OHP etc. Assignments based on course content will be given to the students at the end of each unit/topic and will be evaluated at regular interval. Surprise tests/Quizzes/Tutorials will be conducted. The course includes a ...
... Lectures will be conducted with the aid of multi-media projector, black board, OHP etc. Assignments based on course content will be given to the students at the end of each unit/topic and will be evaluated at regular interval. Surprise tests/Quizzes/Tutorials will be conducted. The course includes a ...
BERKELEY HEIGHTS PUBLIC SCHOOLS
... E. predict electron configurations of atoms F. place electrons in appropriate energy levels and orbitals ...
... E. predict electron configurations of atoms F. place electrons in appropriate energy levels and orbitals ...
preliminary course outline facilitators course description
... (e.g., take the things that you need before entering the classroom, quietly take/leave the seat without interrupting those around you). No cell phones or headphones in class. Browsing facebook, streaming sports, movies and playing games in class is very distracting to other class participants. Pleas ...
... (e.g., take the things that you need before entering the classroom, quietly take/leave the seat without interrupting those around you). No cell phones or headphones in class. Browsing facebook, streaming sports, movies and playing games in class is very distracting to other class participants. Pleas ...
Reactions of common metals and properties of
... resembles that of the proton, H+, but there are more differences than similarities. Hydrogen also forms an anion, the hydride ion, H-, and many metals, including the alkali and alkaline earth metals form salt-like hydrides, such as NaH. Such hydrides have similar crystal structures to alkali halides ...
... resembles that of the proton, H+, but there are more differences than similarities. Hydrogen also forms an anion, the hydride ion, H-, and many metals, including the alkali and alkaline earth metals form salt-like hydrides, such as NaH. Such hydrides have similar crystal structures to alkali halides ...
chemia simr01 en - Leszek Niedzicki
... accumulated in a small volume (not distributed on any neutrons); • In molecules in which hydrogen gives his electron away to atoms with strong affinity towards electrons (e.g. oxygen, nitrogen, fluorine) its electron (although formally shared) is ‘closer’ to the other atom; • Hydrogen is ‘looking’ f ...
... accumulated in a small volume (not distributed on any neutrons); • In molecules in which hydrogen gives his electron away to atoms with strong affinity towards electrons (e.g. oxygen, nitrogen, fluorine) its electron (although formally shared) is ‘closer’ to the other atom; • Hydrogen is ‘looking’ f ...
Spring 2017 - Ventura College Chemistry, Malia Rose-Seisa
... list of recommended questions from the end-of-chapter exercises in the textbook. Your quiz and exam questions will be very similar to these recommended problems. Generally speaking, students who do well in this class are those that spend significant amounts of time outside of class practicing these ...
... list of recommended questions from the end-of-chapter exercises in the textbook. Your quiz and exam questions will be very similar to these recommended problems. Generally speaking, students who do well in this class are those that spend significant amounts of time outside of class practicing these ...