chemical bonds - geraldinescience
... • The forces that hold together the atoms in molecules are called chemical bonds. • Chemical bonds form because of the attraction between positive and negative charges. • Atoms form chemical bonds by either sharing or transferring electrons from one atom to another. • Scientists can study interactio ...
... • The forces that hold together the atoms in molecules are called chemical bonds. • Chemical bonds form because of the attraction between positive and negative charges. • Atoms form chemical bonds by either sharing or transferring electrons from one atom to another. • Scientists can study interactio ...
(3.3 × 10!4) + (2.52 × 10!2) = (3.3 × 10!4) × (2.52 × 10!2)
... All Compounds are made up of molecules or ions. A molecule is the smallest unit of a compound that retains its chemical characteristics. Ionic compounds are described by a “formula unit”. Molecules are described by a “molecular formula”. ...
... All Compounds are made up of molecules or ions. A molecule is the smallest unit of a compound that retains its chemical characteristics. Ionic compounds are described by a “formula unit”. Molecules are described by a “molecular formula”. ...
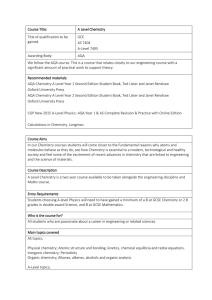
A-level Chemistry
... Students choosing A-level Physics will need to have gained a minimum of a B at GCSE Chemistry or 2 B grades in double award Science, and B at GCSE Mathematics. Who is the course for? All students who are passionate about a career in engineering or related sciences. Main topics covered AS topics; Phy ...
... Students choosing A-level Physics will need to have gained a minimum of a B at GCSE Chemistry or 2 B grades in double award Science, and B at GCSE Mathematics. Who is the course for? All students who are passionate about a career in engineering or related sciences. Main topics covered AS topics; Phy ...
Chemistry 1 Lectures
... – So in ferric chloride (FeCl2) iron ion is Fe2+ – Modern method is to indicate charge on the metal with Roman numerals – So FeCl2 is now named iron(II) chloride ...
... – So in ferric chloride (FeCl2) iron ion is Fe2+ – Modern method is to indicate charge on the metal with Roman numerals – So FeCl2 is now named iron(II) chloride ...
Types of Reactions
... If you can’t come to the Monday Re-Do session, you MUST come before school two days for help. You MUST let me know that you’re interested by Thursday!!!! ...
... If you can’t come to the Monday Re-Do session, you MUST come before school two days for help. You MUST let me know that you’re interested by Thursday!!!! ...
weekly schedule and topics
... This course will discuss the fundamental issues and problems related to a range of topics which are currently at the forefront of heavy inorganic industrial chemistry. The general topics deal with such areas as the development of industrial chemical processes, the environmental protection and air po ...
... This course will discuss the fundamental issues and problems related to a range of topics which are currently at the forefront of heavy inorganic industrial chemistry. The general topics deal with such areas as the development of industrial chemical processes, the environmental protection and air po ...
Catalytic Synthesis of Organophosphorus Compounds from
... largely on the substitutive P-O, P-C coupling of the high-valent phosphorus derivatives (PCl 3, PCl 5, POCl3) with an organic substrates. The emission of a large amount of hydrogen chloride accompanies these reactions with consequent serious environmental and corrosion problems. In this connection, ...
... largely on the substitutive P-O, P-C coupling of the high-valent phosphorus derivatives (PCl 3, PCl 5, POCl3) with an organic substrates. The emission of a large amount of hydrogen chloride accompanies these reactions with consequent serious environmental and corrosion problems. In this connection, ...
Chapter 8 - TeacherWeb
... share one pair of valence electrons A double covalent bond occurs when two atoms share two pairs of valence electrons A triple covalent bond occurs when two atoms share three pairs of covalent bonds ...
... share one pair of valence electrons A double covalent bond occurs when two atoms share two pairs of valence electrons A triple covalent bond occurs when two atoms share three pairs of covalent bonds ...
Chemistry
... addition of HX to unsymmetrical alkenes, preparation of alkenes by dehydration of alcohols; ethyne from calcium dicarbide. Hydrogenation of alkenes. Alkanes and alkenes from petroleum by cracking. ...
... addition of HX to unsymmetrical alkenes, preparation of alkenes by dehydration of alcohols; ethyne from calcium dicarbide. Hydrogenation of alkenes. Alkanes and alkenes from petroleum by cracking. ...
pcc-sio2.alcohol.oxi..
... silica gel residues are then deposited in the solid waste containers for disposal.1 While the conversion of cis,trans-4-tertbutylcyclohexanol to the corresponding ketone gives superior results in terms of an undergraduate protocol, other secondary and primary alcohols (9) will serve as useful substr ...
... silica gel residues are then deposited in the solid waste containers for disposal.1 While the conversion of cis,trans-4-tertbutylcyclohexanol to the corresponding ketone gives superior results in terms of an undergraduate protocol, other secondary and primary alcohols (9) will serve as useful substr ...
Chemistry
... addition of HX to unsymmetrical alkenes, preparation of alkenes by dehydration of alcohols; ethyne from calcium dicarbide. Hydrogenation of alkenes. Alkanes and alkenes from petroleum by cracking. ...
... addition of HX to unsymmetrical alkenes, preparation of alkenes by dehydration of alcohols; ethyne from calcium dicarbide. Hydrogenation of alkenes. Alkanes and alkenes from petroleum by cracking. ...
Chapter 4 REVIEW
... properties because of the different forces involved. For example, while sodium chloride and nickel have nearly identical molar masses, their melting points, conductivity, and solubility in water are quite different. (a) Explain the large difference in melting point between sodium chloride (801˚C) an ...
... properties because of the different forces involved. For example, while sodium chloride and nickel have nearly identical molar masses, their melting points, conductivity, and solubility in water are quite different. (a) Explain the large difference in melting point between sodium chloride (801˚C) an ...
CHEMISTRY IM 06 SYLLABUS 1
... addition of HX to unsymmetrical alkenes, preparation of alkenes by dehydration of alcohols; ethyne from calcium dicarbide. Hydrogenation of alkenes. Alkanes and alkenes from petroleum by cracking. ...
... addition of HX to unsymmetrical alkenes, preparation of alkenes by dehydration of alcohols; ethyne from calcium dicarbide. Hydrogenation of alkenes. Alkanes and alkenes from petroleum by cracking. ...
Deconstructed HS-PS1-2
... trends in the periodic table, and knowledge of the patterns of chemical properties.[Clarification Statement: Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.] [Assessment Boundary: Assessment is limited to chemical rea ...
... trends in the periodic table, and knowledge of the patterns of chemical properties.[Clarification Statement: Examples of chemical reactions could include the reaction of sodium and chlorine, of carbon and oxygen, or of carbon and hydrogen.] [Assessment Boundary: Assessment is limited to chemical rea ...
Properties of Metals vs. Nonmetals vs. Metalloids
... Hydrogen sulfide, a foul-smelling gas, is found in nature in volcanic areas. The balanced chemical equation for the burning of hydrogen sulfide is given below. Interpret this equation in terms of the interaction of the following three relative quantities. 1. The coefficients in this balanced reactio ...
... Hydrogen sulfide, a foul-smelling gas, is found in nature in volcanic areas. The balanced chemical equation for the burning of hydrogen sulfide is given below. Interpret this equation in terms of the interaction of the following three relative quantities. 1. The coefficients in this balanced reactio ...
Properties of Metals vs. Nonmetals vs. Metalloids
... Hydrogen sulfide, a foul-smelling gas, is found in nature in volcanic areas. The balanced chemical equation for the burning of hydrogen sulfide is given below. Interpret this equation in terms of the interaction of the following three relative quantities. 1. The coefficients in this balanced reactio ...
... Hydrogen sulfide, a foul-smelling gas, is found in nature in volcanic areas. The balanced chemical equation for the burning of hydrogen sulfide is given below. Interpret this equation in terms of the interaction of the following three relative quantities. 1. The coefficients in this balanced reactio ...
Investigating Chemistry - Chemistry at Winthrop University
... molecular and has covalent bonds. • When two elements from the upper right corner of the periodic table combine, we use a different system for naming these covalent compounds. • This results in discrete molecules with directional bonds. For example, H2O. • It can also result in an infinite network o ...
... molecular and has covalent bonds. • When two elements from the upper right corner of the periodic table combine, we use a different system for naming these covalent compounds. • This results in discrete molecules with directional bonds. For example, H2O. • It can also result in an infinite network o ...
Organic Chemical Reactions
... thermodynamics and kinetics must be favorable, that is the reaction must have a negative ΔG and should occur in a relatively fast manner. As far as kinetics is concerned the reactants must go through an energy barrier, called the free energy of activation ΔG‡. Once this energy is gained, partial bon ...
... thermodynamics and kinetics must be favorable, that is the reaction must have a negative ΔG and should occur in a relatively fast manner. As far as kinetics is concerned the reactants must go through an energy barrier, called the free energy of activation ΔG‡. Once this energy is gained, partial bon ...
Chemical Reactions
... Synthesis Reactions • A synthesis reaction is a reaction in which two or more substances react to form a single substance • The reactants may be either elements or compounds • The product synthesized is always a compound ...
... Synthesis Reactions • A synthesis reaction is a reaction in which two or more substances react to form a single substance • The reactants may be either elements or compounds • The product synthesized is always a compound ...
Organic chemistry

Organic chemistry is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure includes many physical and chemical methods to determine the chemical composition and the chemical constitution of organic compounds and materials. Study of properties includes both physical properties and chemical properties, and uses similar methods as well as methods to evaluate chemical reactivity, with the aim to understand the behavior of the organic matter in its pure form (when possible), but also in solutions, mixtures, and fabricated forms. The study of organic reactions includes probing their scope through use in preparation of target compounds (e.g., natural products, drugs, polymers, etc.) by chemical synthesis, as well as the focused study of the reactivities of individual organic molecules, both in the laboratory and via theoretical (in silico) study.The range of chemicals studied in organic chemistry include hydrocarbons (compounds containing only carbon and hydrogen), as well as myriad compositions based always on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (these, included in many organic chemicals in biology) and the radiostable elements of the halogens.In the modern era, the range extends further into the periodic table, with main group elements, including:Group 1 and 2 organometallic compounds, i.e., involving alkali (e.g., lithium, sodium, and potassium) or alkaline earth metals (e.g., magnesium)Metalloids (e.g., boron and silicon) or other metals (e.g., aluminium and tin)In addition, much modern research focuses on organic chemistry involving further organometallics, including the lanthanides, but especially the transition metals; (e.g., zinc, copper, palladium, nickel, cobalt, titanium and chromium)Finally, organic compounds form the basis of all earthly life and constitute a significant part of human endeavors in chemistry. The bonding patterns open to carbon, with its valence of four—formal single, double, and triple bonds, as well as various structures with delocalized electrons—make the array of organic compounds structurally diverse, and their range of applications enormous. They either form the basis of, or are important constituents of, many commercial products including pharmaceuticals; petrochemicals and products made from them (including lubricants, solvents, etc.); plastics; fuels and explosives; etc. As indicated, the study of organic chemistry overlaps with organometallic chemistry and biochemistry, but also with medicinal chemistry, polymer chemistry, as well as many aspects of materials science.