Chemistry Mid-Term Review Guide
... • A physical change alters the physical properties of a substance without changing its composition. • A chemical change, also known as a chemical reaction, involves a change in a substance’s composition. ...
... • A physical change alters the physical properties of a substance without changing its composition. • A chemical change, also known as a chemical reaction, involves a change in a substance’s composition. ...
Chemistry: Matter and Change
... • A physical change alters the physical properties of a substance without changing its composition. • A chemical change, also known as a chemical reaction, involves a change in a substance’s composition. ...
... • A physical change alters the physical properties of a substance without changing its composition. • A chemical change, also known as a chemical reaction, involves a change in a substance’s composition. ...
Chemistry B11 Chapter 4 Chemical reactions
... Chemical Equation: we represent a chemical reaction in the form of a chemical equation, using chemical formulas for the reactants and products, and an arrow to indicate the direction in which the reaction proceeds. Note: It is important to show the state of each reactant and product in a chemical eq ...
... Chemical Equation: we represent a chemical reaction in the form of a chemical equation, using chemical formulas for the reactants and products, and an arrow to indicate the direction in which the reaction proceeds. Note: It is important to show the state of each reactant and product in a chemical eq ...
Classifying Reactions: A good summary
... Some other tips and final touches: 1. This all may seem like too much, but remember, you only need to recognize five out of the eight reactions on any given exam. 2. You earn a point for just writing the reactants in chemical form. 3. Don't forget to cancel out spectators. 4. Get familiar with the c ...
... Some other tips and final touches: 1. This all may seem like too much, but remember, you only need to recognize five out of the eight reactions on any given exam. 2. You earn a point for just writing the reactants in chemical form. 3. Don't forget to cancel out spectators. 4. Get familiar with the c ...
Wine Country Lodging near San Luis Obispo CA
... 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 ...
... 3. If both elements are from the same group, the lower element comes first: SiC, BrF3 ...
AP_chemical reaction and quantities
... • The amount of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from ...
... • The amount of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from ...
Gen Chem--Chapter 3 lecture notes.ppt (Read
... from groups 2 or 16, the resulting molecule contains 2 H atoms. The molecule is named according to the previous rules for nonmetallic binary compounds, but the di is omitted: H2S: hydrogen sulfide ...
... from groups 2 or 16, the resulting molecule contains 2 H atoms. The molecule is named according to the previous rules for nonmetallic binary compounds, but the di is omitted: H2S: hydrogen sulfide ...
DEPARTMENT OF CHEMISTRY
... complete mixing, and observe the test tube for the formation of a precipitate (which may appear only as cloudiness in the solution, i.e., turbidity). Record how long it takes for a precipitate to appear; some reactions occur quickly while others take considerable time. After 5 minutes at room temper ...
... complete mixing, and observe the test tube for the formation of a precipitate (which may appear only as cloudiness in the solution, i.e., turbidity). Record how long it takes for a precipitate to appear; some reactions occur quickly while others take considerable time. After 5 minutes at room temper ...
The five main types of redox reactions are combination
... are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always transferred between species. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more compl ...
... are those in which the oxidation states of the reactants change. This occurs because in such reactions, electrons are always transferred between species. Redox reactions take place through either a simple process, such as the burning of carbon in oxygen to yield carbon dioxide (CO2), or a more compl ...
Chemistry: Matter and Change
... • A physical change alters the physical properties of a substance without changing its composition. • A chemical change, also known as a chemical reaction, involves a change in a substance’s composition. ...
... • A physical change alters the physical properties of a substance without changing its composition. • A chemical change, also known as a chemical reaction, involves a change in a substance’s composition. ...
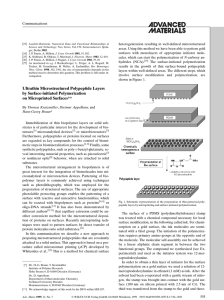
amcommu 555..558 - Leibniz-Institut für Polymerforschung Dresden
... 12-mercaptododecylamine was prepared according to the following procedure: Reaction of 11-bromoundec-1-ene (Lancaster) with thioacetic acid in the presence of AIBN in refluxing CHCl3 yields 11-bromoundecane-1thioacetate. NaCN (2.0 g, 45 mmol) was dissolved in anhydrous DMSO under argon at 100 C and ...
... 12-mercaptododecylamine was prepared according to the following procedure: Reaction of 11-bromoundec-1-ene (Lancaster) with thioacetic acid in the presence of AIBN in refluxing CHCl3 yields 11-bromoundecane-1thioacetate. NaCN (2.0 g, 45 mmol) was dissolved in anhydrous DMSO under argon at 100 C and ...
Atoms and Elements: Are they Related?
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
... • What are the most commonly occurring elements in the food labels? • What items seemed to have the most amount of elements in them? • Can you predict what that means about the food item? • Why do you think the baby formula has such a variety of elements? • Can you predict what the other items on th ...
AP Chemistry Summer Assignment 2016
... a) If 35.0 mL of H2 gas are collected over water when the temperature is 293K, the external pressure is 758 Torr and the vapor pressure of water at that temperature is 20 Torr, how many moles of H2 were ...
... a) If 35.0 mL of H2 gas are collected over water when the temperature is 293K, the external pressure is 758 Torr and the vapor pressure of water at that temperature is 20 Torr, how many moles of H2 were ...
Chemical Equations and Reactions
... acidic solutions to replace the hydrogen in the acid. The products are a metal compound (a salt) and hydrogen gas. ...
... acidic solutions to replace the hydrogen in the acid. The products are a metal compound (a salt) and hydrogen gas. ...
Ch 3 Student.pptx
... 1. The cation is always named first and the anion second. 2. Cation takes its name from the name of the parent element. 3. Anion is named by taking the root of the element name and adding –ide. ...
... 1. The cation is always named first and the anion second. 2. Cation takes its name from the name of the parent element. 3. Anion is named by taking the root of the element name and adding –ide. ...
PSI AP Chemistry Name Unit 4: Chemical Bonding MC Review Part
... 78. The liquefied hydrogen halides have the normal boiling points given below. The relatively high boiling point of HF can be correctly explained by which of the following? (A) HF gas is more ideal. (B) HF is the strongest acid. (C) HF molecules have a smaller dipole moment. (D) HF is much less solu ...
... 78. The liquefied hydrogen halides have the normal boiling points given below. The relatively high boiling point of HF can be correctly explained by which of the following? (A) HF gas is more ideal. (B) HF is the strongest acid. (C) HF molecules have a smaller dipole moment. (D) HF is much less solu ...
Chapter 1.1 –Chemistry is a Physical Science Chemistry is one of
... • Luster ‐ How shiny a substance is. • Malleability ‐ The ability of a substance to be beaten into thin sheets. • Ductility ‐ The ability of a substance to be drawn into thin wires. • Conductivity ‐ The ability of a substance to allow the flow of energy or electricity. • Hardness ‐ How easil ...
... • Luster ‐ How shiny a substance is. • Malleability ‐ The ability of a substance to be beaten into thin sheets. • Ductility ‐ The ability of a substance to be drawn into thin wires. • Conductivity ‐ The ability of a substance to allow the flow of energy or electricity. • Hardness ‐ How easil ...
Single Replacement Reactions - Tri
... • Incomplete combustion occurs when there isn't enough oxygen to allow the fuel (usually a hydrocarbon) to react completely. • Carbon monoxide and pure carbon will be produced in addition to carbon dioxide and water in incomplete combustion. ...
... • Incomplete combustion occurs when there isn't enough oxygen to allow the fuel (usually a hydrocarbon) to react completely. • Carbon monoxide and pure carbon will be produced in addition to carbon dioxide and water in incomplete combustion. ...
PDF
... • The amount of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from o ...
... • The amount of product calculated in the last three examples are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from o ...
PPT
... are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from one container to another and side reactions take place that are ...
... are not the amounts that would be produced if the reactions were actually done in the laboratory. In each case, less product would be obtained than was calculated. There are numerous causes. Some materials are lost during transfers from one container to another and side reactions take place that are ...
Chapter 2 - Cloudfront.net
... that has a unique set of properties. Elements are pure substances made up of only one kind of atom. ...
... that has a unique set of properties. Elements are pure substances made up of only one kind of atom. ...
Formulae/ Equations homework - St Peter the Apostle High School
... Formula Mass 12. Work out the formula mass of the following compounds: (a) (c) (e) (g) ...
... Formula Mass 12. Work out the formula mass of the following compounds: (a) (c) (e) (g) ...
Reduction of nitrogen compounds in oceanic basement and its
... always with mafic glass [7]. The term palagonite is normally used in reference to a bulk sample of metabasite which contains a mixture of palagonitized glass, authigenic minerals like smectite, corrensite, zeolites, carbonates and Fe-Ti oxides and phosphates, as well as primary minerals like plagioc ...
... always with mafic glass [7]. The term palagonite is normally used in reference to a bulk sample of metabasite which contains a mixture of palagonitized glass, authigenic minerals like smectite, corrensite, zeolites, carbonates and Fe-Ti oxides and phosphates, as well as primary minerals like plagioc ...
1 • Venus – Thick, hot, CO 2 – dominated weakly oxidizing
... • H2O photolysis produces hydrogen atoms, part of which are lost to space ("drying" of Mars). • Atmosphere of Mars is simple enough that chemistry models incorporating about 100 reactions work very well. ...
... • H2O photolysis produces hydrogen atoms, part of which are lost to space ("drying" of Mars). • Atmosphere of Mars is simple enough that chemistry models incorporating about 100 reactions work very well. ...
Organic chemistry

Organic chemistry is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure includes many physical and chemical methods to determine the chemical composition and the chemical constitution of organic compounds and materials. Study of properties includes both physical properties and chemical properties, and uses similar methods as well as methods to evaluate chemical reactivity, with the aim to understand the behavior of the organic matter in its pure form (when possible), but also in solutions, mixtures, and fabricated forms. The study of organic reactions includes probing their scope through use in preparation of target compounds (e.g., natural products, drugs, polymers, etc.) by chemical synthesis, as well as the focused study of the reactivities of individual organic molecules, both in the laboratory and via theoretical (in silico) study.The range of chemicals studied in organic chemistry include hydrocarbons (compounds containing only carbon and hydrogen), as well as myriad compositions based always on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (these, included in many organic chemicals in biology) and the radiostable elements of the halogens.In the modern era, the range extends further into the periodic table, with main group elements, including:Group 1 and 2 organometallic compounds, i.e., involving alkali (e.g., lithium, sodium, and potassium) or alkaline earth metals (e.g., magnesium)Metalloids (e.g., boron and silicon) or other metals (e.g., aluminium and tin)In addition, much modern research focuses on organic chemistry involving further organometallics, including the lanthanides, but especially the transition metals; (e.g., zinc, copper, palladium, nickel, cobalt, titanium and chromium)Finally, organic compounds form the basis of all earthly life and constitute a significant part of human endeavors in chemistry. The bonding patterns open to carbon, with its valence of four—formal single, double, and triple bonds, as well as various structures with delocalized electrons—make the array of organic compounds structurally diverse, and their range of applications enormous. They either form the basis of, or are important constituents of, many commercial products including pharmaceuticals; petrochemicals and products made from them (including lubricants, solvents, etc.); plastics; fuels and explosives; etc. As indicated, the study of organic chemistry overlaps with organometallic chemistry and biochemistry, but also with medicinal chemistry, polymer chemistry, as well as many aspects of materials science.