Chemistry for BIOS 302
... temperature range. • The chemical reactions of life are very complex and intertwined. • Mostly involve large molecules (macromolecules) composed of many subunits. ...
... temperature range. • The chemical reactions of life are very complex and intertwined. • Mostly involve large molecules (macromolecules) composed of many subunits. ...
Chemical Reactions
... that the NO3- does not change. Since the nitrate ion and the sodium ion appear on both sides of the equation and they do not seem to do anything in this reaction, they are called a spectator ions. We will actually not even show them in the reaction. ...
... that the NO3- does not change. Since the nitrate ion and the sodium ion appear on both sides of the equation and they do not seem to do anything in this reaction, they are called a spectator ions. We will actually not even show them in the reaction. ...
Werner-type chromium compounds
... acids such as hydrochloric acid. The method of making a novel composition of theory, the compositions‘of this invention may 20 this invention may be generically described as effecting contact in solution between organic be described as Werner complex compounds acido groups containing an —XH radical, ...
... acids such as hydrochloric acid. The method of making a novel composition of theory, the compositions‘of this invention may 20 this invention may be generically described as effecting contact in solution between organic be described as Werner complex compounds acido groups containing an —XH radical, ...
AP Chemistry Unit 1 Essential Questions Screencast 1
... Essential Questions Screencast 1-1 Introduction to the Periodic Table 1. What is an element? 2. How are the symbols for the elements determined? 3. How is the order of the elements determined on the modern periodic table? 4. What are the main regions of the periodic table? 5. What are the special na ...
... Essential Questions Screencast 1-1 Introduction to the Periodic Table 1. What is an element? 2. How are the symbols for the elements determined? 3. How is the order of the elements determined on the modern periodic table? 4. What are the main regions of the periodic table? 5. What are the special na ...
Chemistry Academic v. 2016
... Compare and/or predict the properties (e.g., electron affinity, ionization energy, chemical reactivity, electronegativity, atomic radius) of selected elements by using their locations on the periodic table and known trends. Predict characteristics of an atom or ion based on its location on the perio ...
... Compare and/or predict the properties (e.g., electron affinity, ionization energy, chemical reactivity, electronegativity, atomic radius) of selected elements by using their locations on the periodic table and known trends. Predict characteristics of an atom or ion based on its location on the perio ...
BSC with Chemistry CBCS Syllabus 2016-17
... constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect. Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for different salts. Buffer solutions. Solubility and solubility product of sparingly soluble salts – applications of ...
... constant and ionic product of water. Ionization of weak acids and bases, pH scale, common ion effect. Salt hydrolysis-calculation of hydrolysis constant, degree of hydrolysis and pH for different salts. Buffer solutions. Solubility and solubility product of sparingly soluble salts – applications of ...
Types of Chemical Reactions
... Divide the smallest number of moles of an element into the moles of each element present. Convert the fractional ratios for each element into whole numbers by multiplying all the ratios by the same number. The resulting numbers are the subscripts for the each element in the empirical formula. ...
... Divide the smallest number of moles of an element into the moles of each element present. Convert the fractional ratios for each element into whole numbers by multiplying all the ratios by the same number. The resulting numbers are the subscripts for the each element in the empirical formula. ...
Reactions and Balancing
... Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, double all the coefficients and try again. (Beca ...
... Take one element at a time, working left to right except for H and O. Metals, then nonmetals are a good way, too. Save H for next to last, and O until last. IF everything balances except for O, and there is no way to balance O with a whole number, double all the coefficients and try again. (Beca ...
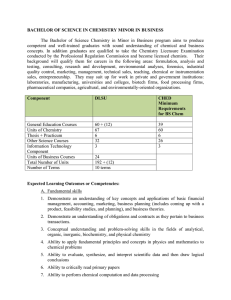
BACHELOR OF SCIENCE IN CHEMISTRY MINOR IN BUSINESS
... organic compounds. The course also covers the physical and chemical properties of alkanes, alkyl halides, alkenes, and alkynes. 3 units Prerequisite: INOCHE2 ORGCHE2 Organic Chemistry 2 for Chemistry and Biochemistry Majors A lecture course covering the structure, nomenclature, physical properties, ...
... organic compounds. The course also covers the physical and chemical properties of alkanes, alkyl halides, alkenes, and alkynes. 3 units Prerequisite: INOCHE2 ORGCHE2 Organic Chemistry 2 for Chemistry and Biochemistry Majors A lecture course covering the structure, nomenclature, physical properties, ...
atoms
... Unfortunately, there is no alternative: The names and charges of the common polyatomic ions must be memorized. (a) Lithium nitrate Lithium (group 1A) forms only the Li+ ion and does not need a Roman numeral. (b) Potassium hydrogen sulfate (potassium bisulfate) Potassium (group 1A) forms only the K+ ...
... Unfortunately, there is no alternative: The names and charges of the common polyatomic ions must be memorized. (a) Lithium nitrate Lithium (group 1A) forms only the Li+ ion and does not need a Roman numeral. (b) Potassium hydrogen sulfate (potassium bisulfate) Potassium (group 1A) forms only the K+ ...
Basic chemistry - Ross University
... Standard conditions: Some thermodynamic properties of a system depend on the conditions that system is in. Chemists have agreed to reference their measurement to a pressure of 1014 hPa (standard atmospheric pressure), a temperature of 25 ◦C (= 298 K) and a concentration of 1 M for all reactants. The ...
... Standard conditions: Some thermodynamic properties of a system depend on the conditions that system is in. Chemists have agreed to reference their measurement to a pressure of 1014 hPa (standard atmospheric pressure), a temperature of 25 ◦C (= 298 K) and a concentration of 1 M for all reactants. The ...
Chemistry - School District of Springfield Township
... • Explain how the relationships of chemical properties of elements are represented in the repeating patterns of the Periodic Table using the periodic law. • Identify and describe the important trends that exist on the Periodic Table and discuss how each trend reflects the elements’ electron configur ...
... • Explain how the relationships of chemical properties of elements are represented in the repeating patterns of the Periodic Table using the periodic law. • Identify and describe the important trends that exist on the Periodic Table and discuss how each trend reflects the elements’ electron configur ...
TDDFT as a tool in chemistry
... Chemistry (from Egyptian kēme (chem), meaning "earth") is the science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions. Historically, modern chemistry evolved out of alchemy following the chemical revolution (1773). Ch ...
... Chemistry (from Egyptian kēme (chem), meaning "earth") is the science concerned with the composition, structure, and properties of matter, as well as the changes it undergoes during chemical reactions. Historically, modern chemistry evolved out of alchemy following the chemical revolution (1773). Ch ...
B.Sc. Industrial Chemistry
... become more comprehensive and relevant. The importance of industrial chemistry hardly needs any emphasis. It basically deals with the development, optimisation and monitoring of various chemical processes used in industry for transforming raw materials etc., into useful commercial products for socie ...
... become more comprehensive and relevant. The importance of industrial chemistry hardly needs any emphasis. It basically deals with the development, optimisation and monitoring of various chemical processes used in industry for transforming raw materials etc., into useful commercial products for socie ...
Chemistry (SPA)
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
... 440 BC, the Greek Leucippus and his pupil Democritus coined the term atomos to describe the smallest particle of matter. It translates to mean something that is indivisible. In the eighteenth century, chemist, John Dalton, revived the term when he suggested that each element was made up of unique at ...
Analytical Chemistry - University of Delhi
... B.Sc Analytical Chemistry Analytical Chemistry is an applied, experimental field of science and is based not only on chemistry, but also on physics, biology, information theory and many fields of technology. It is of fundamental importance not only to all branches of chemistry but also to all biolo ...
... B.Sc Analytical Chemistry Analytical Chemistry is an applied, experimental field of science and is based not only on chemistry, but also on physics, biology, information theory and many fields of technology. It is of fundamental importance not only to all branches of chemistry but also to all biolo ...
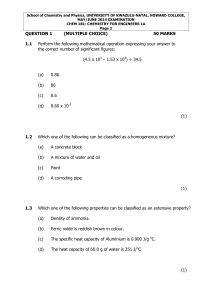
School of Chemistry
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
... School of Chemistry and Physics, UNIVERSITY OF KWAZULU-NATAL, HOWARD COLLEGE, MAY/JUNE 2014 EXAMINATION CHEM 181: CHEMISTRY FOR ENGINEERS 1A Page 10 ...
Parallel Computing in Chemistry
... • An ordinary, real-space LRES calculation would eventually converge, but require >15 periodic images to do so. • The kspace LRES of Ewald is similarly pairwise and similarly scales with N2. • However, the more rapid convergence means we only need 5-7 periodic images. • On the other hand, we now hav ...
... • An ordinary, real-space LRES calculation would eventually converge, but require >15 periodic images to do so. • The kspace LRES of Ewald is similarly pairwise and similarly scales with N2. • However, the more rapid convergence means we only need 5-7 periodic images. • On the other hand, we now hav ...
Chemistry_in_Parallel_Computing_old
... • An ordinary, real-space LRES calculation would eventually converge, but require >15 periodic images to do so. • The kspace LRES of Ewald is similarly pairwise and similarly scales with N2. • However, the more rapid convergence means we only need 5-7 periodic images. • On the other hand, we now hav ...
... • An ordinary, real-space LRES calculation would eventually converge, but require >15 periodic images to do so. • The kspace LRES of Ewald is similarly pairwise and similarly scales with N2. • However, the more rapid convergence means we only need 5-7 periodic images. • On the other hand, we now hav ...
SIDE GROUP ADDITION TO THE POLYCYCLIC AROMATIC
... to previously reported alcohol (”OH) and ketone (>C»O) formation. This work represents the first experimental evidence that ice photochemistry may have contributed to the aromatics bearing carbon and nitrogen containing side groups that are detected in primitive meteorites and interplanetary dust par ...
... to previously reported alcohol (”OH) and ketone (>C»O) formation. This work represents the first experimental evidence that ice photochemistry may have contributed to the aromatics bearing carbon and nitrogen containing side groups that are detected in primitive meteorites and interplanetary dust par ...
Tutorial 7
... cathode surface) is proportional to the quantity of electricity passed in the circuit. ...
... cathode surface) is proportional to the quantity of electricity passed in the circuit. ...
Aqueous Solutions
... nonmetals other than hydrogen – Nomenclature must include prefixes that specify the number of atoms of each element in the compound. • Use the minimum number of prefixes necessary to specify the compound. – Frequently drop the prefix mono- 1 ...
... nonmetals other than hydrogen – Nomenclature must include prefixes that specify the number of atoms of each element in the compound. • Use the minimum number of prefixes necessary to specify the compound. – Frequently drop the prefix mono- 1 ...
Utah - Wavefunction, Inc.
... atoms explains the conservation of matter, since the number of atoms stays the same in a chemical reaction no matter how they are rearranged; the total mass stays the same. Although energy can be absorbed or released in a chemical reaction, the total amount of energy and matter in it remains constan ...
... atoms explains the conservation of matter, since the number of atoms stays the same in a chemical reaction no matter how they are rearranged; the total mass stays the same. Although energy can be absorbed or released in a chemical reaction, the total amount of energy and matter in it remains constan ...
Role of Chemistry in Everyday Life
... we eat have to do with chemistry. They consist of organic compounds like carbohydrates starch and sugar, protein, and lipids. Other nutrients like vitamins and minerals and water are all important chemical compounds. The process of respiration removes oxygen from the environment while adding carbon ...
... we eat have to do with chemistry. They consist of organic compounds like carbohydrates starch and sugar, protein, and lipids. Other nutrients like vitamins and minerals and water are all important chemical compounds. The process of respiration removes oxygen from the environment while adding carbon ...
Organic chemistry

Organic chemistry is a chemistry subdiscipline involving the scientific study of the structure, properties, and reactions of organic compounds and organic materials, i.e., matter in its various forms that contain carbon atoms. Study of structure includes many physical and chemical methods to determine the chemical composition and the chemical constitution of organic compounds and materials. Study of properties includes both physical properties and chemical properties, and uses similar methods as well as methods to evaluate chemical reactivity, with the aim to understand the behavior of the organic matter in its pure form (when possible), but also in solutions, mixtures, and fabricated forms. The study of organic reactions includes probing their scope through use in preparation of target compounds (e.g., natural products, drugs, polymers, etc.) by chemical synthesis, as well as the focused study of the reactivities of individual organic molecules, both in the laboratory and via theoretical (in silico) study.The range of chemicals studied in organic chemistry include hydrocarbons (compounds containing only carbon and hydrogen), as well as myriad compositions based always on carbon, but also containing other elements, especially oxygen, nitrogen, sulfur, phosphorus (these, included in many organic chemicals in biology) and the radiostable elements of the halogens.In the modern era, the range extends further into the periodic table, with main group elements, including:Group 1 and 2 organometallic compounds, i.e., involving alkali (e.g., lithium, sodium, and potassium) or alkaline earth metals (e.g., magnesium)Metalloids (e.g., boron and silicon) or other metals (e.g., aluminium and tin)In addition, much modern research focuses on organic chemistry involving further organometallics, including the lanthanides, but especially the transition metals; (e.g., zinc, copper, palladium, nickel, cobalt, titanium and chromium)Finally, organic compounds form the basis of all earthly life and constitute a significant part of human endeavors in chemistry. The bonding patterns open to carbon, with its valence of four—formal single, double, and triple bonds, as well as various structures with delocalized electrons—make the array of organic compounds structurally diverse, and their range of applications enormous. They either form the basis of, or are important constituents of, many commercial products including pharmaceuticals; petrochemicals and products made from them (including lubricants, solvents, etc.); plastics; fuels and explosives; etc. As indicated, the study of organic chemistry overlaps with organometallic chemistry and biochemistry, but also with medicinal chemistry, polymer chemistry, as well as many aspects of materials science.