Final Exam Review Day 1 - teacherstroh
... If you keep breaking down matter further and further, you will get to something called the… ...
... If you keep breaking down matter further and further, you will get to something called the… ...
The four elements: fire, water, wind, and earth.
... A dense nucleus of positive charge with the electrons circling around it Size scale: if the nucleus of the atom was the size of a tennis ball, the atom would have a diameter over 1 mile. The nearest electron would be .25 mi from the nucleus! If the nucleus was the size of a quarter, the diameter of ...
... A dense nucleus of positive charge with the electrons circling around it Size scale: if the nucleus of the atom was the size of a tennis ball, the atom would have a diameter over 1 mile. The nearest electron would be .25 mi from the nucleus! If the nucleus was the size of a quarter, the diameter of ...
CHEM 1301 FALL 2003 TEST 1 VERSION 1 NO CHEATING
... Atoms can be split into a nucleus and the electrons, but usually the electrons are stuck in the nucleus. Different isotopes of an element contain different numbers of electrons. The three primary sub-atomic particles differ only in their charge. ...
... Atoms can be split into a nucleus and the electrons, but usually the electrons are stuck in the nucleus. Different isotopes of an element contain different numbers of electrons. The three primary sub-atomic particles differ only in their charge. ...
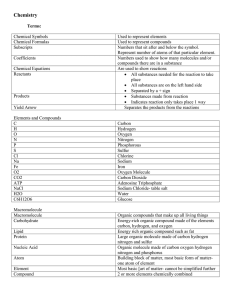
Chemistry Study Guide
... react with other forms of matter. For example, some substances are flammable. If they are heated with oxygen, they will react and burst into flames. The ability of a substance to combine with oxygen is an example of chemical property. ...
... react with other forms of matter. For example, some substances are flammable. If they are heated with oxygen, they will react and burst into flames. The ability of a substance to combine with oxygen is an example of chemical property. ...
Chemistry Study Guide
... react with other forms of matter. For example, some substances are flammable. If they are heated with oxygen, they will react and burst into flames. The ability of a substance to combine with oxygen is an example of chemical property. ...
... react with other forms of matter. For example, some substances are flammable. If they are heated with oxygen, they will react and burst into flames. The ability of a substance to combine with oxygen is an example of chemical property. ...
Unit 2 - Chapter 3 Elements, Atoms, Ions The elements Can we
... • We can take a chunk of matter and break in apart into smaller and smaller pieces, eventually we would get down to individual atoms. Each piece would behave like the original chunk with all of its properties. ...
... • We can take a chunk of matter and break in apart into smaller and smaller pieces, eventually we would get down to individual atoms. Each piece would behave like the original chunk with all of its properties. ...
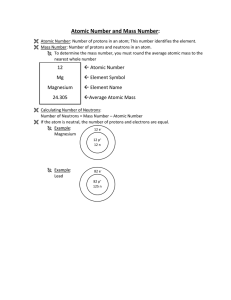
Atomic terms - ATOMIC NUMBER: The number of protons in the
... Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name of the element. Sometimes, the abbreviation is based on a language OTHER THAN ENGLISH! (Example: Na is short for "natrium", the Latin name of s ...
... Atomic number: This is always a whole number. The periodic table is arranged by atomic number! Element symbol: A one or two letter abbreviation for the name of the element. Sometimes, the abbreviation is based on a language OTHER THAN ENGLISH! (Example: Na is short for "natrium", the Latin name of s ...
2. NH3 - Huffman Chemistry Website!
... answers in correct scientific notation (when needed) and significant figures and BOX all answers. ...
... answers in correct scientific notation (when needed) and significant figures and BOX all answers. ...
Goal 4.01
... Given the notation of an element you should be able to determine the number of p, n, and e. The first step is to find the element on the periodic table and determine its atomic number which gives you the number of p. The number of p’s will never change. From there you must determine the number of n ...
... Given the notation of an element you should be able to determine the number of p, n, and e. The first step is to find the element on the periodic table and determine its atomic number which gives you the number of p. The number of p’s will never change. From there you must determine the number of n ...
Atomic Structure/Electrons
... 6. An atom is defined as the smallest part of an element that: a. contains at least one proton, neutron and electron. b. retains the chemical identity of the element. c. can carry an electrical charge. d. is affected in a cathode ray tube. 7. The electrical charges in an atom are located: a. only in ...
... 6. An atom is defined as the smallest part of an element that: a. contains at least one proton, neutron and electron. b. retains the chemical identity of the element. c. can carry an electrical charge. d. is affected in a cathode ray tube. 7. The electrical charges in an atom are located: a. only in ...
Name Date Class Chapter 6 – The Periodic Table Guided Reading
... In Figure 6.10 what does the ability of a helium-filled blimp to rise in the air tell you about the density of helium? ...
... In Figure 6.10 what does the ability of a helium-filled blimp to rise in the air tell you about the density of helium? ...
Chemistry Final Study Guide
... 39. An __________ forms when an atom gains or loses electrons. 40. Elements are organized by atomic number on the __________ __________. 41. The vertical columns are called __________, and elements within each of these have similar properties. 42. The horizontal rows are called __________, and each ...
... 39. An __________ forms when an atom gains or loses electrons. 40. Elements are organized by atomic number on the __________ __________. 41. The vertical columns are called __________, and elements within each of these have similar properties. 42. The horizontal rows are called __________, and each ...
Understanding Atomic Structure of an Element
... element will react with other elements in a reaction -A Valence electron shell is only happy when it contains a full amount of electrons ...
... element will react with other elements in a reaction -A Valence electron shell is only happy when it contains a full amount of electrons ...
O 2 (g)
... • As scientists first began to discover and classify the elements, patterns and similarities were observed in chemical behaviors of certain groups of elements. • Consider the three metals Li, Na, and K ...
... • As scientists first began to discover and classify the elements, patterns and similarities were observed in chemical behaviors of certain groups of elements. • Consider the three metals Li, Na, and K ...
CHAPTER 2: ATOMS, IONS, AND COMPOUNDS
... – Actinide series: Th-Lr, also called transuranium elements, generally all man-made and exist for only very short periods of time before decaying to other elements Periodic Law: ...
... – Actinide series: Th-Lr, also called transuranium elements, generally all man-made and exist for only very short periods of time before decaying to other elements Periodic Law: ...
Periodic Table notes
... b) more mass and is about the same in size c) more mass and is smaller in size d) none of the above ...
... b) more mass and is about the same in size c) more mass and is smaller in size d) none of the above ...
The Basics of Atomic Structure
... are more common than others. • An Ion is an element with a number of electrons that differ from its number of protons. An ion is a charged atom. – Cation is a positive ion – Anion is an negative ion Notice: it’s the number of electrons and neutrons that change, not the atomic number! ...
... are more common than others. • An Ion is an element with a number of electrons that differ from its number of protons. An ion is a charged atom. – Cation is a positive ion – Anion is an negative ion Notice: it’s the number of electrons and neutrons that change, not the atomic number! ...
Unit 7: The Nature of Matter Essential Questions:
... 2. How do we identify different materials? ...
... 2. How do we identify different materials? ...
Chapter 4 Chemical Foundations: Elements, Atoms, and Ions
... • Atoms of a given element are different from those of any other element. – Carbon atoms have different chemical and physical properties than sulfur atoms. ...
... • Atoms of a given element are different from those of any other element. – Carbon atoms have different chemical and physical properties than sulfur atoms. ...
atomic structure (see second part of ppt)
... nucleus could only be located in specific paths called orbitals. This was supported by the line spectra of atoms His model is called the planetary model ...
... nucleus could only be located in specific paths called orbitals. This was supported by the line spectra of atoms His model is called the planetary model ...
Chemistry: Spring Semester Lecture Notes
... equations of the QMM tell us the probability that we will find an electron at a certain distance from the nucleus. ...
... equations of the QMM tell us the probability that we will find an electron at a certain distance from the nucleus. ...
Biochemistry I (CHE 418 / 5418)
... • Second Messenger in cellular signaling. – Nitric oxide is a powerful vasodilator. • Regulates blood pressure, controls muscles that dilate arteries and blood vessels. • Nitric oxide is produced when nitroglycerin is placed under the ...
... • Second Messenger in cellular signaling. – Nitric oxide is a powerful vasodilator. • Regulates blood pressure, controls muscles that dilate arteries and blood vessels. • Nitric oxide is produced when nitroglycerin is placed under the ...
Chemistry DCA Review Sheet
... Chemistry DCA Review Sheet Atoms 1. What are subatomic particles, what are their charges, and where are they found? ...
... Chemistry DCA Review Sheet Atoms 1. What are subatomic particles, what are their charges, and where are they found? ...