TEST II Study Guide-Atomic Theory Honors Chemistry
... Honors Chemistry Atomic Theory (Be able to describe and recognize each scientists’ contribution to the atomic structure. Multiple Choice format) 1. ______________ This person was developed the Law of Conservation of Mass stating “matter cannot be created or destroyed.” 2. _____________ Using the lin ...
... Honors Chemistry Atomic Theory (Be able to describe and recognize each scientists’ contribution to the atomic structure. Multiple Choice format) 1. ______________ This person was developed the Law of Conservation of Mass stating “matter cannot be created or destroyed.” 2. _____________ Using the lin ...
Chemistry PowerPoint
... a. The total mass of the reactants is greater than the total mass of the products b. The total mass of the reactants is less than the total mass of the products c. The total mass of the reactants equals the total mass of the products d. Mass can be created and destroyed ...
... a. The total mass of the reactants is greater than the total mass of the products b. The total mass of the reactants is less than the total mass of the products c. The total mass of the reactants equals the total mass of the products d. Mass can be created and destroyed ...
Goal 4.01
... Then determine the number of electrons within the atom. Draw the 1st orbit and fill that orbit with electrons. Electrons are drawn on the orbit line as a solid circle. Continuing drawing and filling orbits until the correct number of electrons is reached. ...
... Then determine the number of electrons within the atom. Draw the 1st orbit and fill that orbit with electrons. Electrons are drawn on the orbit line as a solid circle. Continuing drawing and filling orbits until the correct number of electrons is reached. ...
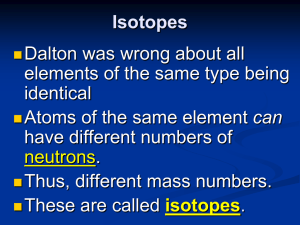
Isotopes
... Atoms of the same element can have different numbers of neutrons. Thus, different mass numbers. These are called isotopes. ...
... Atoms of the same element can have different numbers of neutrons. Thus, different mass numbers. These are called isotopes. ...
Review Periodicity
... 15. The potassium ion is (larger, smaller, the same size ) than the neutral atom. The reason why is electrons were (gained, lost, shared) making the nuclear force increase, decrease, remain the same) 16. The silicon ion is (larger, smaller, the same size) than the neutral atom. The reason why is ele ...
... 15. The potassium ion is (larger, smaller, the same size ) than the neutral atom. The reason why is electrons were (gained, lost, shared) making the nuclear force increase, decrease, remain the same) 16. The silicon ion is (larger, smaller, the same size) than the neutral atom. The reason why is ele ...
Chapter 3
... Electrons can only be at certain energies Electrons must gain a specific amount of energy to move to a higher level, called a quantum ...
... Electrons can only be at certain energies Electrons must gain a specific amount of energy to move to a higher level, called a quantum ...
Diapositiva 1
... – Some particles were deflected at large angles – Discovery: atoms have a very dense positive (+) center, called the nucleus ...
... – Some particles were deflected at large angles – Discovery: atoms have a very dense positive (+) center, called the nucleus ...
Atomic Structure
... II. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. III. Atoms of different elements can physically mix together or can chemically combine in simple whole number ratios to form compounds IV. Chemical reactions occur when atoms are ...
... II. Atoms of the same element are identical. The atoms of any one element are different from those of any other element. III. Atoms of different elements can physically mix together or can chemically combine in simple whole number ratios to form compounds IV. Chemical reactions occur when atoms are ...
SS18A - Atoms, Isotopes and Ions
... In addition to the atomic number, every atom can also be described by its mass number. The mass number is equal to the number of protons and neutrons in the nucleus of an atom. Recall that atoms of the same element have the same number of protons. Atoms of the same element can have different numbers ...
... In addition to the atomic number, every atom can also be described by its mass number. The mass number is equal to the number of protons and neutrons in the nucleus of an atom. Recall that atoms of the same element have the same number of protons. Atoms of the same element can have different numbers ...
PowerPoint - Models of the Atom - A Historical Perspective
... Q- Draw B-R diagrams for the two Li isotopes. A- The atomic # of an element doesn’t change Although the number of neutrons can vary, atoms have definite numbers of protons. ...
... Q- Draw B-R diagrams for the two Li isotopes. A- The atomic # of an element doesn’t change Although the number of neutrons can vary, atoms have definite numbers of protons. ...
ATOMS AND THE PERIODIC TABLE chapter three
... _____ electrons are located in the outermost energy level of an atom. They determine the chemical properties of an element. ...
... _____ electrons are located in the outermost energy level of an atom. They determine the chemical properties of an element. ...
Chapter 5 Review
... As a result of the discovery of the nucleus by Rutherford, what model described an atom? Describe the nucleus of an atom. Know the properties of the electron. Atoms have what charge, and how many protons and electrons? ...
... As a result of the discovery of the nucleus by Rutherford, what model described an atom? Describe the nucleus of an atom. Know the properties of the electron. Atoms have what charge, and how many protons and electrons? ...
John Dalton William Crookes J.J. Thomson Ernest Rutherford
... Scientific Models -Used when objects can’t be studied directly Ex. Stars, Atoms ...
... Scientific Models -Used when objects can’t be studied directly Ex. Stars, Atoms ...
Chapter 10 Power Point - Biloxi Public Schools
... 10-3 Masses of Atoms Isotopes - atoms of the same element with different numbers of neutrons (same # of protons); less common than the main element. The existence of isotopes accounts for the average atomic mass. Average atomic mass - the average mass of the mixture of an element & its isotope ...
... 10-3 Masses of Atoms Isotopes - atoms of the same element with different numbers of neutrons (same # of protons); less common than the main element. The existence of isotopes accounts for the average atomic mass. Average atomic mass - the average mass of the mixture of an element & its isotope ...
Atomic Model Evolution (Webquest)
... Directions: Research and look up the many experiments on that have lead to the current model of the atom. If you feel this sheet will not give enough room you can set this up in your lab-book without using this page. Be as clear and specific as you can. When complete please tape into lab-book for fu ...
... Directions: Research and look up the many experiments on that have lead to the current model of the atom. If you feel this sheet will not give enough room you can set this up in your lab-book without using this page. Be as clear and specific as you can. When complete please tape into lab-book for fu ...
Chem Unit 2 Review Guide ANSWERS
... The Law of Conservation of Mass holds true during chemical reactions, but is not during a nuclear reaction, as mass is converted directly to energy and vice versa. 18.) Define what valence electrons are and how to know how many an element from the representative group has. Valence electrons are an a ...
... The Law of Conservation of Mass holds true during chemical reactions, but is not during a nuclear reaction, as mass is converted directly to energy and vice versa. 18.) Define what valence electrons are and how to know how many an element from the representative group has. Valence electrons are an a ...
the atomic theory
... 5. Neils Bohr 6. nucleus 7. proton 8. neutron 9. electron 10. shell 11. atomic number 12. atomic mass 13. Bohr Model 14. subatomic particle 15. isotope 16. empty bus seat rule B/ THE HISTORY OF THE ATOM: - John Dalton ...
... 5. Neils Bohr 6. nucleus 7. proton 8. neutron 9. electron 10. shell 11. atomic number 12. atomic mass 13. Bohr Model 14. subatomic particle 15. isotope 16. empty bus seat rule B/ THE HISTORY OF THE ATOM: - John Dalton ...
ATOMIC THEORY
... Most of the mass of the atom and all of its positive charge is contained in a tiny core region called the nucleus The nucleus contains protons and neutrons (Chadwick, 1932) that have approximately the same mass The number of protons is the atomic number (Z) The total number of protons and ne ...
... Most of the mass of the atom and all of its positive charge is contained in a tiny core region called the nucleus The nucleus contains protons and neutrons (Chadwick, 1932) that have approximately the same mass The number of protons is the atomic number (Z) The total number of protons and ne ...
History of Atomic Theory
... – Neutron has no charge: “n0” – Mass of n0: 1.67 x 10-24g • Almost the same mass as a proton (both are much larger than the e-) • Described the nucleus and explained isotopes discovered by JJ Thomson in 1913 – Isotopes of the same element have different masses (same # protons but different #s of neu ...
... – Neutron has no charge: “n0” – Mass of n0: 1.67 x 10-24g • Almost the same mass as a proton (both are much larger than the e-) • Described the nucleus and explained isotopes discovered by JJ Thomson in 1913 – Isotopes of the same element have different masses (same # protons but different #s of neu ...
Atomic Theory Notes Page
... measure its position. He found that the electron's position and momentum did indeed obey the uncertainty relation he had derived mathematically Conclusions: Electrons are located in clouds, not neat orbits; tells you where the electron is most likely to be found (a matter of probability). o Chadwi ...
... measure its position. He found that the electron's position and momentum did indeed obey the uncertainty relation he had derived mathematically Conclusions: Electrons are located in clouds, not neat orbits; tells you where the electron is most likely to be found (a matter of probability). o Chadwi ...
Chapter 1 Learning Objective Summary
... and understand fission and fusion Chemical reactions involve the gain, loss, or sharing of the outer electrons, whereas nuclear reactions involve changes to the composition of the nucleus. This means that alchemy is possible (though not economical!), because transmutation of one element into another ...
... and understand fission and fusion Chemical reactions involve the gain, loss, or sharing of the outer electrons, whereas nuclear reactions involve changes to the composition of the nucleus. This means that alchemy is possible (though not economical!), because transmutation of one element into another ...
File
... In KCl, potassium and chlorine always have a ratio of “39.09 to 35.45” or “1.1 to 1” by mass. ...
... In KCl, potassium and chlorine always have a ratio of “39.09 to 35.45” or “1.1 to 1” by mass. ...
Shiny, Happy Pretest - Alex LeMay – Science
... ___29. What is the atomic number? ___30. What is the atomic mass? ___31. How many protons would an atom of this element have? ___32. How many neutrons would an atom of this element likely have? ___33. How many electrons would an atom of this element have? ___34. If an atom of this element had 30 neu ...
... ___29. What is the atomic number? ___30. What is the atomic mass? ___31. How many protons would an atom of this element have? ___32. How many neutrons would an atom of this element likely have? ___33. How many electrons would an atom of this element have? ___34. If an atom of this element had 30 neu ...