Practice Test #2 - smhs
... 15._____ His oil-drop experiment enabled scientists to measure the charge on the electron. 16._____ He concluded that the atom had a small, compact, positively-charged nucleus surrounded by electrons based on his gold-foil experiment. 17._____ He invented the mass spectrograph, an instrument that is ...
... 15._____ His oil-drop experiment enabled scientists to measure the charge on the electron. 16._____ He concluded that the atom had a small, compact, positively-charged nucleus surrounded by electrons based on his gold-foil experiment. 17._____ He invented the mass spectrograph, an instrument that is ...
Chem 200 Dr. Saidane
... regardless of the size of the sample or source of the compound. c) The Law of Multiple Proportions, which states that, if two or more compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element, is always a rat ...
... regardless of the size of the sample or source of the compound. c) The Law of Multiple Proportions, which states that, if two or more compounds are composed of the same two elements, then the ratio of the masses of the second element combined with a certain mass of the first element, is always a rat ...
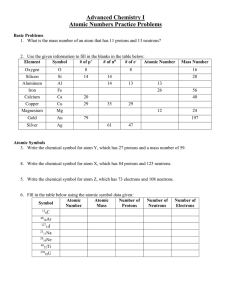
Atomic Numbers Practice Problems
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
... 3. Write the chemical symbol for atom Y, which has 27 protons and a mass number of 59. ...
Atomic Theory
... substances. 2. Pure substances are elements or compounds. 3. Law of constant composition-a compound always has the same composition. ...
... substances. 2. Pure substances are elements or compounds. 3. Law of constant composition-a compound always has the same composition. ...
Chapter 1 Notes
... o The scientists learned that there was something else in an atom that had mass. o That something else had no charge. • Neutron – electrically neutral particle that has the same mass as a proton and is found in an atom’s nucleus. o It took another 20 years for scientists to prove this theory. ...
... o The scientists learned that there was something else in an atom that had mass. o That something else had no charge. • Neutron – electrically neutral particle that has the same mass as a proton and is found in an atom’s nucleus. o It took another 20 years for scientists to prove this theory. ...
Atoms, Molecules and Ions In This Chapter
... – An element is composed of identical atoms with fixed, identical properties and masses. – Compounds are formed by the combination of atoms of 2 or more different elements in a fixed whole number ratio. – A chemical reaction involves a combination, separation or rearrangement of atoms. Atoms are nei ...
... – An element is composed of identical atoms with fixed, identical properties and masses. – Compounds are formed by the combination of atoms of 2 or more different elements in a fixed whole number ratio. – A chemical reaction involves a combination, separation or rearrangement of atoms. Atoms are nei ...
Getting to Know: Periodic Table
... an investigation. A data table allows scientists to collect data in an organized way. It makes it easier to identify patterns and trends and to compare the results of multiple trials, for example. A table uses a logical system to organize information. Back in the 1800s, scientists were researching t ...
... an investigation. A data table allows scientists to collect data in an organized way. It makes it easier to identify patterns and trends and to compare the results of multiple trials, for example. A table uses a logical system to organize information. Back in the 1800s, scientists were researching t ...
The Atom - Taylorsville
... Five main points of Dalton's atomic theory 1. Elements are made of tiny particles called atoms. 2. All atoms of a given element are identical. ...
... Five main points of Dalton's atomic theory 1. Elements are made of tiny particles called atoms. 2. All atoms of a given element are identical. ...
Nuclear Chemistry PowerPoint
... reactions. Although many people think of the sun as a large fireball, the sun (and all stars) are actually enormous fusion reactors. Stars are primarily gigantic balls of hydrogen gas under tremendous pressure due to gravitational forces. Hydrogen molecules are fused into helium and heavier elements ...
... reactions. Although many people think of the sun as a large fireball, the sun (and all stars) are actually enormous fusion reactors. Stars are primarily gigantic balls of hydrogen gas under tremendous pressure due to gravitational forces. Hydrogen molecules are fused into helium and heavier elements ...
Atomic theory
... different properties, including mass and chemical reactivity. 4. Atoms are not changed by chemical reactions, but merely rearranged into different compounds. ...
... different properties, including mass and chemical reactivity. 4. Atoms are not changed by chemical reactions, but merely rearranged into different compounds. ...
SNC 1D chem chpt2
... to represent different chemicals are the same in each language, although we do call elements different names. We use a standard atomic notation to represent elements. Mass # is written above, atomic # below and the symbol in large letters to the left. ...
... to represent different chemicals are the same in each language, although we do call elements different names. We use a standard atomic notation to represent elements. Mass # is written above, atomic # below and the symbol in large letters to the left. ...
Review: theory vs law the atomic theory contributions of early scientists
... Neutron Heavy (similar to nucleus protons) Oct 711:36 AM ...
... Neutron Heavy (similar to nucleus protons) Oct 711:36 AM ...
File
... The smallest atomic unit The process of combining two light nuclei to form a heavier more stable nucleus The process of using a neutron to split a heavy nucleus into two nuclei with smaller mass numbers Brittle versus soft Stretchable Metal-like but does not contain all metal characteristics A posit ...
... The smallest atomic unit The process of combining two light nuclei to form a heavier more stable nucleus The process of using a neutron to split a heavy nucleus into two nuclei with smaller mass numbers Brittle versus soft Stretchable Metal-like but does not contain all metal characteristics A posit ...
Atomic Number, Mass Number, and Isotopes
... number is the total number of protons and neutrons that make up the nucleus of an isotope. For example, carbon-14, commonly used to date biological objects (up to approximately 50,000 years old), has six protons (Z=6) and eight neutrons. To determine the number of neutrons in an isotope: Mass Number ...
... number is the total number of protons and neutrons that make up the nucleus of an isotope. For example, carbon-14, commonly used to date biological objects (up to approximately 50,000 years old), has six protons (Z=6) and eight neutrons. To determine the number of neutrons in an isotope: Mass Number ...
Test Review Answers File
... a. Protons = 15 b. Neutrons = 16 c. Electrons = 15 20. Which part of the atom was discovered as a result of the Gold Foil experiment? ...
... a. Protons = 15 b. Neutrons = 16 c. Electrons = 15 20. Which part of the atom was discovered as a result of the Gold Foil experiment? ...
Name ____ Date
... I can relate the mass and number of atoms in a mole to the molar mass and Avogadro's number Objective 3 ...
... I can relate the mass and number of atoms in a mole to the molar mass and Avogadro's number Objective 3 ...
Thomson`s Atom
... • In the mass spectrometer, atoms enter the device and are ionized. • The ions are then accelerated through a magnetic field which bends the ion paths into a semicircular shape. • The radius of this path is dependent upon the mass of the particle (with all other factors such as speed and charge bein ...
... • In the mass spectrometer, atoms enter the device and are ionized. • The ions are then accelerated through a magnetic field which bends the ion paths into a semicircular shape. • The radius of this path is dependent upon the mass of the particle (with all other factors such as speed and charge bein ...
Elements Compounds Mixtures
... Subscript- tells the number of atoms in a compound ex. H2O CO2 Coefficient- tells the number of molecules ex. 2H2O Chemical Equation- describes the chemical reaction using symbols and formulas ...
... Subscript- tells the number of atoms in a compound ex. H2O CO2 Coefficient- tells the number of molecules ex. 2H2O Chemical Equation- describes the chemical reaction using symbols and formulas ...
1st Term Review
... 14. Based on the gold foil experiment, what did Rutherford conclude about the atom? 15. An atom of chromium-60 contains how many protons, neutron and electrons? 16. What is the difference between a compound and an element? 17. What is the electron configuration of a neutral calcium atom? 18. Atomic ...
... 14. Based on the gold foil experiment, what did Rutherford conclude about the atom? 15. An atom of chromium-60 contains how many protons, neutron and electrons? 16. What is the difference between a compound and an element? 17. What is the electron configuration of a neutral calcium atom? 18. Atomic ...