TEK 8.5D: Chemical Formulas
... HC 2 H 3 O 2 (vinegar), NaHCO 3 (baking soda), NH 4 (ammonia), and C 6 H 12 O 6 (glucose). ...
... HC 2 H 3 O 2 (vinegar), NaHCO 3 (baking soda), NH 4 (ammonia), and C 6 H 12 O 6 (glucose). ...
Midterm Review.ppt - Chemistry R: 4(AE)
... mass of water in a sample of BaCl2 * 2H2O. The accepted value is 14.8%. What was the ...
... mass of water in a sample of BaCl2 * 2H2O. The accepted value is 14.8%. What was the ...
Chemistry Study Guide What is matter made of? Matter is anything
... group, or family. All of the elements in a column belong to the same group. Elements in the same group have ...
... group, or family. All of the elements in a column belong to the same group. Elements in the same group have ...
Atomic mass - cloudfront.net
... table could have called it the Periodic Table of tlie Atorns but they did not have a firm understanding of atoms atthattirne. Sincetheywere workingwith actual samples of elements such as copper, mercury, sulfur, etc., the_v called it the periodic table of the elements. ...
... table could have called it the Periodic Table of tlie Atorns but they did not have a firm understanding of atoms atthattirne. Sincetheywere workingwith actual samples of elements such as copper, mercury, sulfur, etc., the_v called it the periodic table of the elements. ...
Chapter 4: The Structure of the Atom Early Ideas about Matter Name
... Oil drop experiment (gravity, e– charge, and charged plates) α – particle / gold foil ...
... Oil drop experiment (gravity, e– charge, and charged plates) α – particle / gold foil ...
Ch 1.1 ppt
... protons and neutrons in an atom. This is the relative mass of the element compared to carbon as a standard. ...
... protons and neutrons in an atom. This is the relative mass of the element compared to carbon as a standard. ...
c) C2 Glossary Topic 1
... The number of objects of a particular kind in a sample (shown as a percentage of the total number of objects) ...
... The number of objects of a particular kind in a sample (shown as a percentage of the total number of objects) ...
3-ELEMENTS AND THE ATOMIC MODEL. C4.8A Identify the
... Identify properties of common families of elements. Identify properties of common periods on the periodic table. Explain the history and organization of the periodic table. C4.8e Write the complete electron configuration of elements in the first three rows of the periodic table. C4.8g Predict oxidat ...
... Identify properties of common families of elements. Identify properties of common periods on the periodic table. Explain the history and organization of the periodic table. C4.8e Write the complete electron configuration of elements in the first three rows of the periodic table. C4.8g Predict oxidat ...
a) air c) milk f) beer
... Dalton’s Atomic Theory (in his own words): Atomic Theory – An explanation of the structure of matter in terms of different combinations of very small particles. ...
... Dalton’s Atomic Theory (in his own words): Atomic Theory – An explanation of the structure of matter in terms of different combinations of very small particles. ...
Slide 1
... mass of water in a sample of BaCl2 * 2H2O. The accepted value is 14.8%. What was the student's ...
... mass of water in a sample of BaCl2 * 2H2O. The accepted value is 14.8%. What was the student's ...
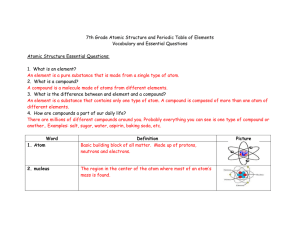
7th Grade Atomic Structure and Periodic Table of Elements
... A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is composed of more than one atom of different elements. 4. How are compounds a part of our daily li ...
... A compound is a molecule made of atoms from different elements. 3. What is the difference between and element and a compound? An element is a substance that contains only one type of atom. A compound is composed of more than one atom of different elements. 4. How are compounds a part of our daily li ...
Atoms
... Ernest Rutherford – 1911 Gold Foil Experiment ‘As if you had fired a 15 – inch shell at a piece of tissue paper And it came back to hit you.’ ...
... Ernest Rutherford – 1911 Gold Foil Experiment ‘As if you had fired a 15 – inch shell at a piece of tissue paper And it came back to hit you.’ ...
Atomic Structure
... Atoms cannot be subdivided, created, or destroyed in ordinary chemical reactions. However, these changes CAN occur in nuclear reactions! Atoms of an element have a characteristic average mass which is unique to that element. Atoms of any one element differ in properties from atoms of another ele ...
... Atoms cannot be subdivided, created, or destroyed in ordinary chemical reactions. However, these changes CAN occur in nuclear reactions! Atoms of an element have a characteristic average mass which is unique to that element. Atoms of any one element differ in properties from atoms of another ele ...
Atomic Structure
... When scientists design models of atoms, they usually show a simplified version of the atom's nucleus and its subatomic particles. The nucleus is made up of protons and neutrons (picture red and blue gumballs stuck together) with electrons moving at high speeds around the outside of the nucleus (imag ...
... When scientists design models of atoms, they usually show a simplified version of the atom's nucleus and its subatomic particles. The nucleus is made up of protons and neutrons (picture red and blue gumballs stuck together) with electrons moving at high speeds around the outside of the nucleus (imag ...
Document
... In this reaction two light atomic nuclei, when they are very close to each other, fuse together to form a single heavier nucleus of a new element. The process is exothermic (release of energy). The nuclear fusions occur at only very high temperatures. When 2 hydrogen nuclei fuse together by nuclear ...
... In this reaction two light atomic nuclei, when they are very close to each other, fuse together to form a single heavier nucleus of a new element. The process is exothermic (release of energy). The nuclear fusions occur at only very high temperatures. When 2 hydrogen nuclei fuse together by nuclear ...
Atoms - ChemistryatBiotech
... lost or gained with oxidation numbers (also known as charges) Ions are charged particles –when an atom has too many or too few electrons to be neutral No change to the nucleus Proton and neutrons stay the same number. ...
... lost or gained with oxidation numbers (also known as charges) Ions are charged particles –when an atom has too many or too few electrons to be neutral No change to the nucleus Proton and neutrons stay the same number. ...
the Atom
... tiny, indivisible, indestructible particles. Each one has a certain mass, size, and chemical behavior that was determined by what kind of element they were. A Summary of Dalton’s Atomic Theory: ...
... tiny, indivisible, indestructible particles. Each one has a certain mass, size, and chemical behavior that was determined by what kind of element they were. A Summary of Dalton’s Atomic Theory: ...
Atoms and the Periodic Table Notes
... 3) Atoms of different elements ______________________ together in simple proportions to create a compound. 4) In a chemical ________________________, atoms are ________________________, but not ...
... 3) Atoms of different elements ______________________ together in simple proportions to create a compound. 4) In a chemical ________________________, atoms are ________________________, but not ...
Atoms overview quiz
... You cannot ever know the exact location of an electron. There will always be some margin of error because they are so small and even light can knock them around. Equations can tell you places you should find them, but never the exact spot at one moment in time. QUESTION 10: Atoms in the same family ...
... You cannot ever know the exact location of an electron. There will always be some margin of error because they are so small and even light can knock them around. Equations can tell you places you should find them, but never the exact spot at one moment in time. QUESTION 10: Atoms in the same family ...
Atomic structure and periodic table notes sheet
... Atomic Structure and Periodic Table Notes 1. What are Atoms? ___________________________________________ ___________________________________________ 2. 400 BC- _________________ was the first scientist to suggest that all matter is composed of tiny, indivisible particles called atoms. (Atomos) 3. El ...
... Atomic Structure and Periodic Table Notes 1. What are Atoms? ___________________________________________ ___________________________________________ 2. 400 BC- _________________ was the first scientist to suggest that all matter is composed of tiny, indivisible particles called atoms. (Atomos) 3. El ...
- Elliott Hudson College
... Atoms consist of a central ____________ containing protons and ___________. The nucleus is _______ compared to the size of the whole atom. The nucleus is surrounded by ___________ in energy levels (also called _________). Atoms have no electric charge because they contain the same number of protons ...
... Atoms consist of a central ____________ containing protons and ___________. The nucleus is _______ compared to the size of the whole atom. The nucleus is surrounded by ___________ in energy levels (also called _________). Atoms have no electric charge because they contain the same number of protons ...
Matt Knorr 2/3/2014 Summary: This lesson will explore the smallest
... finally have students write a short summary of any known stable or unstable isotopes of their selected element for submission in a later class. This summary should include labeled sketches of at least one stable and one unstable isotope of student’s selected element. ...
... finally have students write a short summary of any known stable or unstable isotopes of their selected element for submission in a later class. This summary should include labeled sketches of at least one stable and one unstable isotope of student’s selected element. ...