Atomic Timeline There are small, negatively charged particles inside
... Atomic Timeline The table below contains a number of statements connected to major discoveries in the development of the atomic theory. ...
... Atomic Timeline The table below contains a number of statements connected to major discoveries in the development of the atomic theory. ...
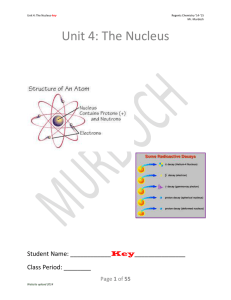
Unit 4: The Nucleus
... expected to calculate this energy in Regents Chemistry, but you do need to understand that ...
... expected to calculate this energy in Regents Chemistry, but you do need to understand that ...
Atoms
... Is the number of protons in the nucleus of each atom of the element In a periodic table the atomic number is above the symbol of the element http://education.jlab.org/qa/pen_number. html ...
... Is the number of protons in the nucleus of each atom of the element In a periodic table the atomic number is above the symbol of the element http://education.jlab.org/qa/pen_number. html ...
Class 11 Class 12 The p- Block Element • Group13 (B to Tl
... elements of group 2. From left to right in the period, the magnitude of nuclear charge increases but the electrons are added to, the same shell. These electrons do not screen each other, therefore, the electrons experience greater nuclear charge. • In other words, effective nuclear ...
... elements of group 2. From left to right in the period, the magnitude of nuclear charge increases but the electrons are added to, the same shell. These electrons do not screen each other, therefore, the electrons experience greater nuclear charge. • In other words, effective nuclear ...
Chemical Reactions
... Identify the type of reaction for each of the following synthesis or decomposition reactions, and write the balanced equation: Nitrogen and oxygen react to form nitrogen ...
... Identify the type of reaction for each of the following synthesis or decomposition reactions, and write the balanced equation: Nitrogen and oxygen react to form nitrogen ...
Introductory Chemistry, 2nd Edition Nivaldo Tro
... • The number of protons in the nucleus of an atom is called the atomic number. Z is the short-hand designation for the atomic number. Because each element’s atoms have a unique number of protons, each element can be identified by its atomic number. The elements are arranged on the Periodic Tab ...
... • The number of protons in the nucleus of an atom is called the atomic number. Z is the short-hand designation for the atomic number. Because each element’s atoms have a unique number of protons, each element can be identified by its atomic number. The elements are arranged on the Periodic Tab ...
EARLY ATOMIC THEORY AND STRUCTURE
... Dalton – no positive matter in his model Thomson – positive matter is distributed throughout the atom Rutherford – positive matter is concentrated in a small central nucleus ...
... Dalton – no positive matter in his model Thomson – positive matter is distributed throughout the atom Rutherford – positive matter is concentrated in a small central nucleus ...
atomic number
... B: atoms/molecules are bombarded by a stream of high energy electrons to produce positive ions, mostly w/ a 1+ charge. These electrons collide w/ electrons in the particle knocking them out and leaving a positive ...
... B: atoms/molecules are bombarded by a stream of high energy electrons to produce positive ions, mostly w/ a 1+ charge. These electrons collide w/ electrons in the particle knocking them out and leaving a positive ...
Unit 2 Atomic Theories and Structures Packet
... 14)______________________ A positively charged particle. 15)______________________ Chemical compounds have the same elements in exactly the same ratios. 16)______________________ This English scientist is credited with discovering the neutron. 17)______________________ It represents the number of pr ...
... 14)______________________ A positively charged particle. 15)______________________ Chemical compounds have the same elements in exactly the same ratios. 16)______________________ This English scientist is credited with discovering the neutron. 17)______________________ It represents the number of pr ...
Research Papers-Quantum Theory / Particle Physics/Download/6583
... numbers of their farthest orbits. Shells of the same ranges compose Groups and distinguish themselves by fullness of their orbits. The known chemical elements make up 14 Groups that have total of 14 orbits, reserved for electronic groupings of different numbers of electrons. The first of these orbit ...
... numbers of their farthest orbits. Shells of the same ranges compose Groups and distinguish themselves by fullness of their orbits. The known chemical elements make up 14 Groups that have total of 14 orbits, reserved for electronic groupings of different numbers of electrons. The first of these orbit ...
Chapter 4- Elements and the Periodic Table
... mass of just one proton. A proton and a neutron are about equal in mass. Together, the protons and neutrons make up nearly all the mass of an atom. Figure 8 compares the charges and masses of the three atomic particles. Atoms are too small to be described easily by everyday units of mass, such as gr ...
... mass of just one proton. A proton and a neutron are about equal in mass. Together, the protons and neutrons make up nearly all the mass of an atom. Figure 8 compares the charges and masses of the three atomic particles. Atoms are too small to be described easily by everyday units of mass, such as gr ...
atomic mass - Belle Vernon Area School District
... and 6 neutrons), and call it 1 atomic mass unit (amu) protons and neutrons have a mass of just over 1 amu electrons have a mass of 0.00055 amu too small to be relevant to the total mass of the atom ...
... and 6 neutrons), and call it 1 atomic mass unit (amu) protons and neutrons have a mass of just over 1 amu electrons have a mass of 0.00055 amu too small to be relevant to the total mass of the atom ...
Unit 2 - Solon City Schools
... 14)______________________ A positively charged particle. 15)______________________ Chemical compounds have the same elements in exactly the same ratios. 16)______________________ This English scientist is credited with discovering the neutron. 17)______________________ It represents the number of pr ...
... 14)______________________ A positively charged particle. 15)______________________ Chemical compounds have the same elements in exactly the same ratios. 16)______________________ This English scientist is credited with discovering the neutron. 17)______________________ It represents the number of pr ...
Assignment # 6 Atomic Structure Drill
... 1. Use atomic number and mass number of an element to find the number of protons, electrons, and neutrons in a particular atom. 2. State how isotopes of an atom differ. 3. Interpret and write isotopic notation. ...
... 1. Use atomic number and mass number of an element to find the number of protons, electrons, and neutrons in a particular atom. 2. State how isotopes of an atom differ. 3. Interpret and write isotopic notation. ...
Problem Solving Drill - Rapid Learning Center
... 3. A complete chemical symbol displays all the information about an atom’s protons, neutrons and electrons. Such a symbol contains an element symbol, surrounded by the atomic number, the mass number of the isotope and the charge of the ion. For the following, give the number of protons (p), neutrons ...
... 3. A complete chemical symbol displays all the information about an atom’s protons, neutrons and electrons. Such a symbol contains an element symbol, surrounded by the atomic number, the mass number of the isotope and the charge of the ion. For the following, give the number of protons (p), neutrons ...
Chapter 2
... but different mass numbers (meaning the same number of protons but different numbers of neutrons) The atomic number determines the identity of the element Atoms of the same element with differing mass numbers are termed isotopes Malone and Dolter - Basic Concepts of Chemistry 9e ...
... but different mass numbers (meaning the same number of protons but different numbers of neutrons) The atomic number determines the identity of the element Atoms of the same element with differing mass numbers are termed isotopes Malone and Dolter - Basic Concepts of Chemistry 9e ...
Chapter 2 - Chemistry
... We can see in the formulas (and models shown) that CO2 has 2 oxygen atoms per carbon atom while CO has 1 oxygen atom per carbon atom. This is reflected in the mass ratio! ...
... We can see in the formulas (and models shown) that CO2 has 2 oxygen atoms per carbon atom while CO has 1 oxygen atom per carbon atom. This is reflected in the mass ratio! ...
Chapter 2 - Chemistry
... Dalton’s atomic theory (1808) Each element is made of tiny indestructible particles called atoms. 2. All atoms of a given element have the same mass and physical/chemical properties that distinguish them from atoms of other elements. 3. Atoms combine in simple whole number ratios to form compounds. ...
... Dalton’s atomic theory (1808) Each element is made of tiny indestructible particles called atoms. 2. All atoms of a given element have the same mass and physical/chemical properties that distinguish them from atoms of other elements. 3. Atoms combine in simple whole number ratios to form compounds. ...
chemistry - billpalmer
... 1) Matter is composed of small particles called atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combi ...
... 1) Matter is composed of small particles called atoms 2) All atoms of the same element are identical; different atoms are different 3) Atoms cannot be subdivided, created, or destroyed 4) atoms combine in simple whole number ratios to form chemical compounds 5) In chemical reactions, atoms are combi ...
Chemistry Semester 1 Exam Review Study Island
... 83. Polyatomic ions are a group of A. ionically bonded atoms that dissociate when dissolved in water or an acid. B. covalently bonded atoms that act like a single atom when combining with other atoms. C. elements that have a similar number of protons but a varying number of neutrons. D. elements tha ...
... 83. Polyatomic ions are a group of A. ionically bonded atoms that dissociate when dissolved in water or an acid. B. covalently bonded atoms that act like a single atom when combining with other atoms. C. elements that have a similar number of protons but a varying number of neutrons. D. elements tha ...