Counting Atoms
... describe how they apply to isotopes. • Given the identity of a nuclide, determine its number of protons, neutrons, and electrons. • Define mole, Avogadro’s number, and molar mass, and state how all three are related. • Solve problems involving mass in grams, amount in moles, and number of atoms of a ...
... describe how they apply to isotopes. • Given the identity of a nuclide, determine its number of protons, neutrons, and electrons. • Define mole, Avogadro’s number, and molar mass, and state how all three are related. • Solve problems involving mass in grams, amount in moles, and number of atoms of a ...
sec 3- Counting atoms - Nutley Public Schools
... describe how they apply to isotopes. • Given the identity of a nuclide, determine its number of protons, neutrons, and electrons. • Define mole, Avogadro’s number, and molar mass, and state how all three are related. • Solve problems involving mass in grams, amount in moles, and number of atoms of a ...
... describe how they apply to isotopes. • Given the identity of a nuclide, determine its number of protons, neutrons, and electrons. • Define mole, Avogadro’s number, and molar mass, and state how all three are related. • Solve problems involving mass in grams, amount in moles, and number of atoms of a ...
U N I 1. laboratory tools and chemistry techniques.
... smaller mass, it also has a smaller volume, so the density can be the same. 6. m DV 10.5 g/cm3 • 238.1 cm3 ...
... smaller mass, it also has a smaller volume, so the density can be the same. 6. m DV 10.5 g/cm3 • 238.1 cm3 ...
Chapter 17 Resource: Properties of Atoms and the Periodic Table
... Can you determine whether or not a solution is active? Can you put metals in order based on their activities? ...
... Can you determine whether or not a solution is active? Can you put metals in order based on their activities? ...
atm-atomic structure - Discovery Education
... requires imagination and concentration. Ask students to keep this in mind as they begin the program. ...
... requires imagination and concentration. Ask students to keep this in mind as they begin the program. ...
TOPIC 2. THE STRUCTURE OF ATOMS
... Note how the electron arrangement in the Na+ ion is the same as for the Ne atom which has atomic number 10, one smaller than the Na atom. All the other members of the first group of elements in Table 2 (the alkali metals) also have just one more electron than a noble gas atom, and they all behave as ...
... Note how the electron arrangement in the Na+ ion is the same as for the Ne atom which has atomic number 10, one smaller than the Na atom. All the other members of the first group of elements in Table 2 (the alkali metals) also have just one more electron than a noble gas atom, and they all behave as ...
High School Knowledge Exam – Study Guide
... Chemical Change examples: Reactions between chemicals, burning (fire reacts with something), color change (caused by reaction b/w chemicals) Dalton’s Atomic Theory 1) All matter is made up of very small, discrete particles called atoms 2) All atoms of a given element are identical, and the atoms of ...
... Chemical Change examples: Reactions between chemicals, burning (fire reacts with something), color change (caused by reaction b/w chemicals) Dalton’s Atomic Theory 1) All matter is made up of very small, discrete particles called atoms 2) All atoms of a given element are identical, and the atoms of ...
Structure of the Atom
... Or, we can write distribution of electrons in a sodium atom as 2, 8, 1. Question 2: If K and L shells of an atom are full, then what would be the total number of electrons in the atom? Answer: The maximum number of electrons that can occupy K and L-shells of an atom are 2 and 8 respectively. Therefo ...
... Or, we can write distribution of electrons in a sodium atom as 2, 8, 1. Question 2: If K and L shells of an atom are full, then what would be the total number of electrons in the atom? Answer: The maximum number of electrons that can occupy K and L-shells of an atom are 2 and 8 respectively. Therefo ...
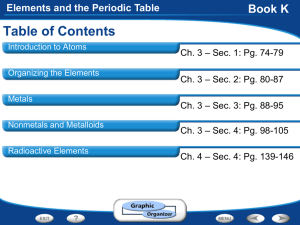
Elements and the Periodic Table
... nonmetals. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...
... nonmetals. Atoms of these elements easily form compounds by sharing or gaining one electron when reacting with atoms of other elements. ...
PSN Chapter 14 Multi-format Test.tst
... repeated. This pattern is called ____________________ Short Answer 13. Two particles found in the nucleus of most atoms have masses equivalent to one atomic mass unit, or 1 amu. Name the particles. ...
... repeated. This pattern is called ____________________ Short Answer 13. Two particles found in the nucleus of most atoms have masses equivalent to one atomic mass unit, or 1 amu. Name the particles. ...
Redox
... (Cl-) and chlorate(V) ions (ClO3-). Deduce the two half-equations for this reaction, and hence derive an overall equation. Chlorine is changing its oxidation state from 0 in Cl2 to -1 in Cl- and therefore needs to gain 1e-. ½ Cl2 + eClChlorine is changing its oxidation state from 0 in Cl2 to +5 in C ...
... (Cl-) and chlorate(V) ions (ClO3-). Deduce the two half-equations for this reaction, and hence derive an overall equation. Chlorine is changing its oxidation state from 0 in Cl2 to -1 in Cl- and therefore needs to gain 1e-. ½ Cl2 + eClChlorine is changing its oxidation state from 0 in Cl2 to +5 in C ...
The Atom
... chemical and physical properties. For example, the most common oxygen isotope has 8 neutrons in the nucleus. Other isotopes of oxygen have 9 or 10 neutrons. All three isotopes are colorless, odorless gases at room temperature. Each isotope has the chemical property of combining with a substance as i ...
... chemical and physical properties. For example, the most common oxygen isotope has 8 neutrons in the nucleus. Other isotopes of oxygen have 9 or 10 neutrons. All three isotopes are colorless, odorless gases at room temperature. Each isotope has the chemical property of combining with a substance as i ...
Build an Atom Scripted - UTeach Outreach
... but they would eventually become known as electrons. Since the metal atoms themselves were electrically neutral, the negative charge on the electrons meant that there must also be some kind of positively charged particles within an atom, which led to the 1917 discovery of the proton, and in turn to ...
... but they would eventually become known as electrons. Since the metal atoms themselves were electrically neutral, the negative charge on the electrons meant that there must also be some kind of positively charged particles within an atom, which led to the 1917 discovery of the proton, and in turn to ...
TOPIC 2. THE STRUCTURE OF ATOMS
... distributed around the nucleus, but never actually being within the nucleus. Now the normal laws of electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of elect ...
... distributed around the nucleus, but never actually being within the nucleus. Now the normal laws of electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of elect ...
Build an Atom Scripted
... but they would eventually become known as electrons. Since the metal atoms themselves were electrically neutral, the negative charge on the electrons meant that there must also be some kind of positively charged particles within an atom, which led to the 1917 discovery of the proton, and in turn to ...
... but they would eventually become known as electrons. Since the metal atoms themselves were electrically neutral, the negative charge on the electrons meant that there must also be some kind of positively charged particles within an atom, which led to the 1917 discovery of the proton, and in turn to ...
Periodic table Periodic Trends
... An oxide is formed from the combination of an element with oxygen Use the charge of a metal cation to deduce the chemical formula of a metal oxide: • Na+ combines with O2- to form Na2O • Ca2+ combines with O2- to form CaO • Al3+ combines with O2- to form Al2O3 Oxides of metals are basic: they react ...
... An oxide is formed from the combination of an element with oxygen Use the charge of a metal cation to deduce the chemical formula of a metal oxide: • Na+ combines with O2- to form Na2O • Ca2+ combines with O2- to form CaO • Al3+ combines with O2- to form Al2O3 Oxides of metals are basic: they react ...
TOPIC 2. THE STRUCTURE OF ATOMS
... distributed around the nucleus, but never actually being within the nucleus. Now the normal laws of electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of elect ...
... distributed around the nucleus, but never actually being within the nucleus. Now the normal laws of electrostatics would require the electrons to collapse into the nucleus due to the attraction of the protons. Because this does not happen, there must be other laws which govern the behaviour of elect ...
Problem Solving Drill - Rapid Learning Center
... Good job! A weighted average is closest to the one that occurs most often. B. Incorrect! A weighted average is closest to the one that occurs most often. C. Incorrect! A weighted average is closest to the one that occurs most often. ...
... Good job! A weighted average is closest to the one that occurs most often. B. Incorrect! A weighted average is closest to the one that occurs most often. C. Incorrect! A weighted average is closest to the one that occurs most often. ...
Problem Solving Drill - Rapid Learning Center
... Good job! A weighted average is closest to the one that occurs most often. B. Incorrect! A weighted average is closest to the one that occurs most often. C. Incorrect! A weighted average is closest to the one that occurs most often. ...
... Good job! A weighted average is closest to the one that occurs most often. B. Incorrect! A weighted average is closest to the one that occurs most often. C. Incorrect! A weighted average is closest to the one that occurs most often. ...
9th class bridge course 74-112
... Atoms of different elements are different in all respects. Atoms combine in small whole numbers to form compound atoms (molecules). Atom is the smallest unit of matter which takes part in a chemical reaction. All the points put forward in Dalton’s atomic theory have been contradicted by modern resea ...
... Atoms of different elements are different in all respects. Atoms combine in small whole numbers to form compound atoms (molecules). Atom is the smallest unit of matter which takes part in a chemical reaction. All the points put forward in Dalton’s atomic theory have been contradicted by modern resea ...
TOPIC 2. THE STRUCTURE OF ATOMS
... common, but atoms from the fifth group of elements in Table 2 starting with nitrogen which are all 3 electrons short of the noble gas structure, can in some instances gain ...
... common, but atoms from the fifth group of elements in Table 2 starting with nitrogen which are all 3 electrons short of the noble gas structure, can in some instances gain ...
Chapter 2 "Elements, Atoms, and the Periodic Table"
... fluorine compounds in the drinking water were discovered to be the cause of both these effects. Research continued, and in the 1930s, the US Public Health Service found that low levels of fluorine in water would provide the benefit of resisting decay without discoloring teeth. The protective effects ...
... fluorine compounds in the drinking water were discovered to be the cause of both these effects. Research continued, and in the 1930s, the US Public Health Service found that low levels of fluorine in water would provide the benefit of resisting decay without discoloring teeth. The protective effects ...
Stars and Elements
... • Chemistry is broadly defined as the study of matter. • Chemists typically focus on two important characteristics of matter in their work: composition and structure. Composition is defined as the elements that make up a chemical compound. Structure is defined as the way those elements are arranged ...
... • Chemistry is broadly defined as the study of matter. • Chemists typically focus on two important characteristics of matter in their work: composition and structure. Composition is defined as the elements that make up a chemical compound. Structure is defined as the way those elements are arranged ...
Atomic Theory PowerPoint Notes
... 1. Electrons assume only certain orbits around the nucleus. These orbits are stable and called "stationary" orbits. 2. Each orbit has an energy level associated with it. For example the orbit closest to the nucleus has an energy E1, the next closest E2 and so on. 3. Light is emitted when an electron ...
... 1. Electrons assume only certain orbits around the nucleus. These orbits are stable and called "stationary" orbits. 2. Each orbit has an energy level associated with it. For example the orbit closest to the nucleus has an energy E1, the next closest E2 and so on. 3. Light is emitted when an electron ...