Atoms and Elements
... • What is the atomic mass of silicon, Si? • How many protons does a chlorine atom have? • How many electrons does a neutral neon atom have? • Will an atom with 6 protons, 6 neutrons and 6 electrons be electrically neutral? • Will an atom with 27 protons, 32 neutrons, and 27 electrons be electrically ...
... • What is the atomic mass of silicon, Si? • How many protons does a chlorine atom have? • How many electrons does a neutral neon atom have? • Will an atom with 6 protons, 6 neutrons and 6 electrons be electrically neutral? • Will an atom with 27 protons, 32 neutrons, and 27 electrons be electrically ...
LESSON PLAN Subject: Chemistry Topic: Matter matters!
... A. Read the text provided and underline in red the sentences, which state what makes the atoms of two different elements different from each other. B. Underline in blue the sentences, which state why all atoms of an element do not have the same number of neutrons All matter, such as solids, liquids ...
... A. Read the text provided and underline in red the sentences, which state what makes the atoms of two different elements different from each other. B. Underline in blue the sentences, which state why all atoms of an element do not have the same number of neutrons All matter, such as solids, liquids ...
Lecture 2 - TCD Chemistry
... Atoms can be subdivided, modified or transferred into new atoms by physical reactions (nuclear reactions) Reason: energies are sufficiently high ...
... Atoms can be subdivided, modified or transferred into new atoms by physical reactions (nuclear reactions) Reason: energies are sufficiently high ...
cp chemistry midterm exam review topics and problems
... c. neutral group of atoms held together by covalent bonds. d. neutral group of atoms held together by ionic bonds. ____ 104. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Av ...
... c. neutral group of atoms held together by covalent bonds. d. neutral group of atoms held together by ionic bonds. ____ 104. What principle states that atoms tend to form compounds so that each atom can have eight electrons in its outermost energy level? a. rule of eights c. configuration rule b. Av ...
Worksheet 2 Structure of matter Task 2.1.
... A. Read the text provided and underline in red the sentences, which state what makes the atoms of two different elements different from each other. B. Underline in blue the sentences, which state why all atoms of an element do not have the same number of neutrons All matter, such as solids, liquids ...
... A. Read the text provided and underline in red the sentences, which state what makes the atoms of two different elements different from each other. B. Underline in blue the sentences, which state why all atoms of an element do not have the same number of neutrons All matter, such as solids, liquids ...
- Catalyst
... Question 7: Fill in the blanks of the statements below with the words in the box. Note, you will only use each word once. 1. A positively charged ion is a(n) ____________________. 2. A proton has a +1 _______________________________. 3. Like charges _________________________ each other. ...
... Question 7: Fill in the blanks of the statements below with the words in the box. Note, you will only use each word once. 1. A positively charged ion is a(n) ____________________. 2. A proton has a +1 _______________________________. 3. Like charges _________________________ each other. ...
AP Biology
... Life requires about 25 chemical elements (pp. 27-28, TABLE 2.1) Carbon, oxygen, hydrogen, and nitrogen make up approximately 96% of living matter. ATOMS AND MOLECULES Atomic structure determines the behavior of an element (pp. 28-33, FIGURE 2.10) An atom is the smallest unit of an element. An at ...
... Life requires about 25 chemical elements (pp. 27-28, TABLE 2.1) Carbon, oxygen, hydrogen, and nitrogen make up approximately 96% of living matter. ATOMS AND MOLECULES Atomic structure determines the behavior of an element (pp. 28-33, FIGURE 2.10) An atom is the smallest unit of an element. An at ...
Chapter 2
... Life requires about 25 chemical elements (pp. 27-28, TABLE 2.1) Carbon, oxygen, hydrogen, and nitrogen make up approximately 96% of living matter. ATOMS AND MOLECULES Atomic structure determines the behavior of an element (pp. 28-33, FIGURE 2.10) An atom is the smallest unit of an element. An at ...
... Life requires about 25 chemical elements (pp. 27-28, TABLE 2.1) Carbon, oxygen, hydrogen, and nitrogen make up approximately 96% of living matter. ATOMS AND MOLECULES Atomic structure determines the behavior of an element (pp. 28-33, FIGURE 2.10) An atom is the smallest unit of an element. An at ...
Chapter 4: The Structure of the Atom
... With each expulsion of an alpha particle from the atom’s nucleus the atom loses 4 units of mass & 2 protons (+2 charged ...
... With each expulsion of an alpha particle from the atom’s nucleus the atom loses 4 units of mass & 2 protons (+2 charged ...
Document
... kind of atoms called isotopes that have slightly different masses but same nuclear charges. In modern atomic theory, the postulate has been amended to read: "Elements are characterized by the nuclear charge of their atoms". ...
... kind of atoms called isotopes that have slightly different masses but same nuclear charges. In modern atomic theory, the postulate has been amended to read: "Elements are characterized by the nuclear charge of their atoms". ...
Element - Faculty
... Chemical formulas can be determined by measuring the mass of each element present in a sample of the compound. The mass of each element (grams) is converted to number of moles, or molecules of each element presenting the compound. You will need to do such calculations in order to determine the amoun ...
... Chemical formulas can be determined by measuring the mass of each element present in a sample of the compound. The mass of each element (grams) is converted to number of moles, or molecules of each element presenting the compound. You will need to do such calculations in order to determine the amoun ...
Chem101 - Lecture 2 Elements Elements as Pure
... - Chlorine (Cl) also has two naturally occurring isotopes: one has 18 neutrons and represents 75.78% of all naturally occurring chlorine, the other has 20 neutrons and represents the remaining 24.22% of all naturally occurring chlorine. - Magnesium (Mg) has three naturally occurring isotopes: one ha ...
... - Chlorine (Cl) also has two naturally occurring isotopes: one has 18 neutrons and represents 75.78% of all naturally occurring chlorine, the other has 20 neutrons and represents the remaining 24.22% of all naturally occurring chlorine. - Magnesium (Mg) has three naturally occurring isotopes: one ha ...
Hewitt/Lyons/Suchocki/Yeh, Conceptual Integrated Science
... observed collisions between visible particles and invisible atoms (Brownian motion)—later confirmed by Einstein as evidence for the existence of atoms. ...
... observed collisions between visible particles and invisible atoms (Brownian motion)—later confirmed by Einstein as evidence for the existence of atoms. ...
Chapter 2: Chemical Basis of Life
... of either the components or the processes of living things without using the biochemist's terms. For example, 96% of the human body is made up of just four major elements. Chemical reactions that hold atoms together do so by forming chemical bonds—these include ionic (electrovalent) bonds, covalent ...
... of either the components or the processes of living things without using the biochemist's terms. For example, 96% of the human body is made up of just four major elements. Chemical reactions that hold atoms together do so by forming chemical bonds—these include ionic (electrovalent) bonds, covalent ...
Chapter 2 power point File
... A mole is equal to an elements atoms mass (the big number in the square of the elements periodic box) A mole is also equal to the sum of all the elements in a molecule or a compound Avogadro’s number is 6.022 X 1023 atoms This number represents the number of atoms that can be counted in one mole of ...
... A mole is equal to an elements atoms mass (the big number in the square of the elements periodic box) A mole is also equal to the sum of all the elements in a molecule or a compound Avogadro’s number is 6.022 X 1023 atoms This number represents the number of atoms that can be counted in one mole of ...
Atomic structure
... no matter how much or how little of the compound you have. These proportions are in _____________________; for example every water molecule has two hydrogen atoms for each oxygen atom (H2O). You would not say that there is 1 hydrogen for each 1/2 oxygen. So when elements combine during chemical reac ...
... no matter how much or how little of the compound you have. These proportions are in _____________________; for example every water molecule has two hydrogen atoms for each oxygen atom (H2O). You would not say that there is 1 hydrogen for each 1/2 oxygen. So when elements combine during chemical reac ...
Intro to Moles Notes File
... of manganese is 54.94 g/mol. Using a conversion factor to convert from moles to mass 1. From the periodic table, find the atomic mass of the element. (in amu) 2. Determine the molar mass of the element. (g/mol) 3. Create a conversion factor to do your calculations Molar mass ...
... of manganese is 54.94 g/mol. Using a conversion factor to convert from moles to mass 1. From the periodic table, find the atomic mass of the element. (in amu) 2. Determine the molar mass of the element. (g/mol) 3. Create a conversion factor to do your calculations Molar mass ...
Grade 11 Unit 4 - Amazon Web Services
... Follow these directions and record your data in the table. Place a check in the box when the step is completed. ❏ 1. Get some samples of pure elements from the shelf or from your teacher. ❏ 2. From the observations you can make and with the use of a handbook, reference text, and Science LIFEPAC 1103 ...
... Follow these directions and record your data in the table. Place a check in the box when the step is completed. ❏ 1. Get some samples of pure elements from the shelf or from your teacher. ❏ 2. From the observations you can make and with the use of a handbook, reference text, and Science LIFEPAC 1103 ...
Chemistry: Matter and Change
... by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – H2O2 and H2O – Copper(I) chloride and copper(II) chloride ...
... by a combination of the same elements, different masses of one element combine with the same relative mass of the other element in whole number ratios. – H2O2 and H2O – Copper(I) chloride and copper(II) chloride ...
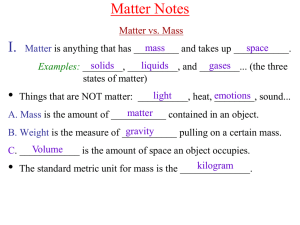
Chem A Week 2 Matter Notes
... chromatography. It is the physical separation of a mixture into its individual components. It involves using a solvent to pass through the mixture. What solvent should I use? The solvent used depends upon the solubility of the mixture you are trying to separate. Examples are water, isopropyl alcohol ...
... chromatography. It is the physical separation of a mixture into its individual components. It involves using a solvent to pass through the mixture. What solvent should I use? The solvent used depends upon the solubility of the mixture you are trying to separate. Examples are water, isopropyl alcohol ...
Atomic Structure Study Guide
... Gold Foil Experiment: Marsden aimed a narrow beam of alpha particles at the gold. The screen around the gold was made of a material that produced a flash of light when struck by a fast-moving alpha particle. By observing the flash, Marsden could figure out the path of an alpha particle after it pass ...
... Gold Foil Experiment: Marsden aimed a narrow beam of alpha particles at the gold. The screen around the gold was made of a material that produced a flash of light when struck by a fast-moving alpha particle. By observing the flash, Marsden could figure out the path of an alpha particle after it pass ...
Unit 3 Powerpoint
... All are solids at room temp (except Mercury, which is a liquid) Metals tend to have low ionization energies, and typically lose electrons (i.e. are oxidized) when they undergo chemical reactions Alkali metals are always 1+ (lose the electron in s subshell) Alkaline earth metals are always 2+ (lose b ...
... All are solids at room temp (except Mercury, which is a liquid) Metals tend to have low ionization energies, and typically lose electrons (i.e. are oxidized) when they undergo chemical reactions Alkali metals are always 1+ (lose the electron in s subshell) Alkaline earth metals are always 2+ (lose b ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.