Linking Asteroids and Meteorites through Reflectance
... Atoms are made up of 3 types of particles Protons – positive charge (+1) Electrons – negative charge (-1) Neutrons – neutral charge (no charge) Protons and Neutrons are found in the nucleus ...
... Atoms are made up of 3 types of particles Protons – positive charge (+1) Electrons – negative charge (-1) Neutrons – neutral charge (no charge) Protons and Neutrons are found in the nucleus ...
chapter 2-1 - Doral Academy Preparatory
... Radioactivity—process of spontaneous atomic decay What can we use this for? ...
... Radioactivity—process of spontaneous atomic decay What can we use this for? ...
GY 111 Lecture Note Series Elemental Chemistry
... Those particular things were called atoms. At last count, there were just over 100 elements (although several of them were produced in labs rather than found in nature). Each has a specific chemical symbol. The elements can combine through various chemical reactions to for compounds. For example: wa ...
... Those particular things were called atoms. At last count, there were just over 100 elements (although several of them were produced in labs rather than found in nature). Each has a specific chemical symbol. The elements can combine through various chemical reactions to for compounds. For example: wa ...
3 Atoms
... o Summarize the five essential points of Dalton’s atomic theory o Explain the relationship between Dalton’s atomic theory and the law of conservation of mass, the law of definite proportions, and the law of multiple proportions o Summarize the experiment of Thompson that led to the discovery of the ...
... o Summarize the five essential points of Dalton’s atomic theory o Explain the relationship between Dalton’s atomic theory and the law of conservation of mass, the law of definite proportions, and the law of multiple proportions o Summarize the experiment of Thompson that led to the discovery of the ...
Document
... An industrially important element contains 26 electrons and rusts in the presence of air and moisture. Identify the element. ...
... An industrially important element contains 26 electrons and rusts in the presence of air and moisture. Identify the element. ...
Isotopes
... • Atoms of the same element with different atomic masses are called isotopes. • Atoms of the same element have the same properties. • But what causes the chemical properties of an atom? • So atoms of different isotopes of an element must have the same number of protons and electrons. • Whatever cau ...
... • Atoms of the same element with different atomic masses are called isotopes. • Atoms of the same element have the same properties. • But what causes the chemical properties of an atom? • So atoms of different isotopes of an element must have the same number of protons and electrons. • Whatever cau ...
Dalton, Plum Pudding, and Rutherford`s Atomic Theories (Models) 9
... 1. To learn about Dalton’s theory of atoms 2. To understand and illustrate the Law of constant composition 3. To learn how a formula describes a compound’s composition 4. To learn about the internal parts of an atom 5. To learn about The Plum Pudding model of an atom 6. To understand Rutherford’s ex ...
... 1. To learn about Dalton’s theory of atoms 2. To understand and illustrate the Law of constant composition 3. To learn how a formula describes a compound’s composition 4. To learn about the internal parts of an atom 5. To learn about The Plum Pudding model of an atom 6. To understand Rutherford’s ex ...
Atomic Theory
... What would the charge be on a sodium ion? Since sodium in in Group IA it is a metal and so would LOSE an electron You can tell how many would be lost by the group number Group 1A elements lose 1 electron ...
... What would the charge be on a sodium ion? Since sodium in in Group IA it is a metal and so would LOSE an electron You can tell how many would be lost by the group number Group 1A elements lose 1 electron ...
File
... 21. List two Noble Gases and explain why they are unique. Neon and Argon have full valence electron levels making them very stable 22. List two Alkali Metals and explain why they are unique. Lithium and sodium are the most reactive elements since they only have one valence electron in their outer en ...
... 21. List two Noble Gases and explain why they are unique. Neon and Argon have full valence electron levels making them very stable 22. List two Alkali Metals and explain why they are unique. Lithium and sodium are the most reactive elements since they only have one valence electron in their outer en ...
Blue File
... Over the years Many Scientists have put forward ‘models’ that they have arrived at from their research of what they understand an atom to look like……. Firstly came : ‘J.J Thompson’s ‘Plum Pudding ‘ model which showed that ‘atoms were tiny balls of positive charge with tiny negative particles stuck i ...
... Over the years Many Scientists have put forward ‘models’ that they have arrived at from their research of what they understand an atom to look like……. Firstly came : ‘J.J Thompson’s ‘Plum Pudding ‘ model which showed that ‘atoms were tiny balls of positive charge with tiny negative particles stuck i ...
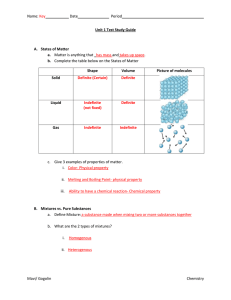
Unit 1 Test Study Guide KEY
... In a liquid the force of attraction between the particles is weaker than it is in the solid. It is still strong enough that the particles are held close to each other but they are now free to move. A gas takes up a lot more space (occupies a greater volume) than the boiling liquid it came from. This ...
... In a liquid the force of attraction between the particles is weaker than it is in the solid. It is still strong enough that the particles are held close to each other but they are now free to move. A gas takes up a lot more space (occupies a greater volume) than the boiling liquid it came from. This ...
Matter and Atoms - davis.k12.ut.us
... Every element is composed of tiny particles. These particles are called atoms. All atoms of a given element are identical, atoms of different elements have different properties. Atoms of an element are NOT changed into atoms of another element through chemical processes. Matter can not be created no ...
... Every element is composed of tiny particles. These particles are called atoms. All atoms of a given element are identical, atoms of different elements have different properties. Atoms of an element are NOT changed into atoms of another element through chemical processes. Matter can not be created no ...
Chapter 18 Notes
... Chemical symbols are the one or two letter abbreviations for elements, either one capital letter or two letters, first capital, second lower case. Atomic Components: Atom: smallest piece of matter that retains the element’s properties, composed of particles called protons, neutrons and electrons Nuc ...
... Chemical symbols are the one or two letter abbreviations for elements, either one capital letter or two letters, first capital, second lower case. Atomic Components: Atom: smallest piece of matter that retains the element’s properties, composed of particles called protons, neutrons and electrons Nuc ...
T1 Final Study Guide - District 196 e
... 24. How did Demitri Mendeleev arrange the elements on the periodic table? Atomic Mass 25. How did Henry Mosely arrange the elements on the period table? Atomic Number 26. What are the periodic trends for electronegativity, ionization energy, and atomic radius? Electronegativity- Increases across the ...
... 24. How did Demitri Mendeleev arrange the elements on the periodic table? Atomic Mass 25. How did Henry Mosely arrange the elements on the period table? Atomic Number 26. What are the periodic trends for electronegativity, ionization energy, and atomic radius? Electronegativity- Increases across the ...
Atomic theory
... different properties, including mass and chemical reactivity. 4. Atoms are not changed by chemical reactions, but merely rearranged into different compounds. ...
... different properties, including mass and chemical reactivity. 4. Atoms are not changed by chemical reactions, but merely rearranged into different compounds. ...
Study Guide Answer Key
... 5. H2O - Compound 6. NaCl- Compound 7. Salt Water – Homogenous Mixture 8. Caesar Salad- Heterogenous Mixture ...
... 5. H2O - Compound 6. NaCl- Compound 7. Salt Water – Homogenous Mixture 8. Caesar Salad- Heterogenous Mixture ...
Lec: Periodic Table of Elements
... WebElements: A Periodic Table on the Web Periodic Table of Elements: Videos Interactive Periodic Table of Elements ...
... WebElements: A Periodic Table on the Web Periodic Table of Elements: Videos Interactive Periodic Table of Elements ...
Year 9 Science Revision Unit: Elements NGA PUMOTU O
... a pure substance composed of more than one type of atom two or more atoms chemically combined in a fixed ratio two or more elements or compounds that are easily separated can be seen or tested without changing the substance the smallest particle of matter to exist on its own a negative sub-atomic pa ...
... a pure substance composed of more than one type of atom two or more atoms chemically combined in a fixed ratio two or more elements or compounds that are easily separated can be seen or tested without changing the substance the smallest particle of matter to exist on its own a negative sub-atomic pa ...
atomic structure - IGCSE STUDY BANK
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
... Isotopes are atoms of the same element with different numbers of neutrons. This gives each isotope of the element a different mass or nucleon number but being the same element they have the same atomic or proton number. There are small physical differences between the isotopes eg the heavier isotope ...
Atomic mass - cloudfront.net
... the periodic table, the nurnber of electrons in an atom always equals the number of protons in the nucieus. But this is not true for neutrons. Atoms of the same element can have different numbers of neutrons than protons. Atoms of the same element with different rrumbers of neutrons are called isoto ...
... the periodic table, the nurnber of electrons in an atom always equals the number of protons in the nucieus. But this is not true for neutrons. Atoms of the same element can have different numbers of neutrons than protons. Atoms of the same element with different rrumbers of neutrons are called isoto ...
Document
... Elements on the left of a period have a lower atomic mass than elements on the right of a period. Elements at the bottom of a group have more protons than elements at the top of the group. ...
... Elements on the left of a period have a lower atomic mass than elements on the right of a period. Elements at the bottom of a group have more protons than elements at the top of the group. ...
introductory chemistry
... Elements are substances that contain only one type of atom. Hydrogen gas is an element as it contains only hydrogen atoms. Compounds contain the atoms of two or more different elements joined together. Water is a compound that consists of hydrogen and oxygen atoms joined together. There are nearly 1 ...
... Elements are substances that contain only one type of atom. Hydrogen gas is an element as it contains only hydrogen atoms. Compounds contain the atoms of two or more different elements joined together. Water is a compound that consists of hydrogen and oxygen atoms joined together. There are nearly 1 ...
Atomic Structure 1
... What happens if... • An atom gains or loses electrons? – you get an ION…a charged particle ...
... What happens if... • An atom gains or loses electrons? – you get an ION…a charged particle ...
DEVELOPMENT OF THE ATOMIC MODEL
... All matter is made of tiny particles called “atoms” Atoms are indivisible and indestructible Atoms of the same element are identical Atoms of different elements differ in some fundamental way Atoms combine in simple whole number ratios to form compounds ...
... All matter is made of tiny particles called “atoms” Atoms are indivisible and indestructible Atoms of the same element are identical Atoms of different elements differ in some fundamental way Atoms combine in simple whole number ratios to form compounds ...
Atomic Structure Test Review 2016
... You may need to check your notes for some definitions. Remember, resources are on ItsLearning. ...
... You may need to check your notes for some definitions. Remember, resources are on ItsLearning. ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.