PowerPoint - Models of the Atom - A Historical Perspective
... Isotopes and Radioisotopes Isotopes: Atoms of the same element that have different numbers of neutrons – Due to isotopes, mass #s are not round #s. – E.g. Li (6.9) is made up of both 6Li and 7Li. – Often, at least one isotope is unstable.It breaks down, releasing radioactivity.These types of isotop ...
... Isotopes and Radioisotopes Isotopes: Atoms of the same element that have different numbers of neutrons – Due to isotopes, mass #s are not round #s. – E.g. Li (6.9) is made up of both 6Li and 7Li. – Often, at least one isotope is unstable.It breaks down, releasing radioactivity.These types of isotop ...
File
... extremely reactive • fluorine is the most reactive • Noble gases (Group 18) – extremely low chemical reactivity • ASSIGN p.g. 55 - #1,4,5 and p.g. 67 #3 ...
... extremely reactive • fluorine is the most reactive • Noble gases (Group 18) – extremely low chemical reactivity • ASSIGN p.g. 55 - #1,4,5 and p.g. 67 #3 ...
No Slide Title
... elements, including man-made. Of all the elements on the periodic table, only 92 are found in nature and only 25 are necessary to life. ...
... elements, including man-made. Of all the elements on the periodic table, only 92 are found in nature and only 25 are necessary to life. ...
Chapter 2
... from charged atoms or groups of atoms called ions – Metal atoms tend to lose electrons: result is a positive charge (cation) – Nonmetals tend to gain electrons: result is a negative charge (anion) • Sodium loses one electron to become Na+ • Calcium loses two electrons to become Ca2+ • May consist of ...
... from charged atoms or groups of atoms called ions – Metal atoms tend to lose electrons: result is a positive charge (cation) – Nonmetals tend to gain electrons: result is a negative charge (anion) • Sodium loses one electron to become Na+ • Calcium loses two electrons to become Ca2+ • May consist of ...
Review-Semester Final (Part I)
... 25. The ___________________in an atom are the subatomic particles involved in a chemical reaction. (protons, neutrons, valance electrons, electrons) ...
... 25. The ___________________in an atom are the subatomic particles involved in a chemical reaction. (protons, neutrons, valance electrons, electrons) ...
Metals, Nonmetals, and Metalloids (Vocabulary)
... are arranged by properties and are represented by one or two letter chemical symbols. ...
... are arranged by properties and are represented by one or two letter chemical symbols. ...
Chapter 4 Chemical Foundations: Elements, Atoms, and Ions
... Dalton’s Atomic Theory 1. Elements are made of tiny particles called atoms. 2. All atoms of a given element are identical (not exactly; isotopes) 3. The atoms of a given element are different from those of any other element. 4. Atoms of one element can combine with atoms of other elements to form co ...
... Dalton’s Atomic Theory 1. Elements are made of tiny particles called atoms. 2. All atoms of a given element are identical (not exactly; isotopes) 3. The atoms of a given element are different from those of any other element. 4. Atoms of one element can combine with atoms of other elements to form co ...
Chemical Element
... resulted in the production of hydrogen and helium isotopes, as well as very minuscule amounts (on the order of 10-10) of lithium and beryllium. No heavier elements were produced. As a result, the primordial abundance of atoms consisted of roughly 75% 1H, 25% 4He, and 0.01% deuterium. Subsequent enri ...
... resulted in the production of hydrogen and helium isotopes, as well as very minuscule amounts (on the order of 10-10) of lithium and beryllium. No heavier elements were produced. As a result, the primordial abundance of atoms consisted of roughly 75% 1H, 25% 4He, and 0.01% deuterium. Subsequent enri ...
Development of the Periodic Table
... ◦ Atom: the smallest particle of an element that keeps the properties of that element. (Greek: atomos = indivisible) ...
... ◦ Atom: the smallest particle of an element that keeps the properties of that element. (Greek: atomos = indivisible) ...
Final Exam review semester 1
... 1. Ninety-nine percent of all the matter that can be observed in the universe exists as 2. Hydrochloric acid, HCl, is added to solid NaOH. After the reaction is complete, NaCl dissolved in water remains. What are the products of this chemical reaction? ...
... 1. Ninety-nine percent of all the matter that can be observed in the universe exists as 2. Hydrochloric acid, HCl, is added to solid NaOH. After the reaction is complete, NaCl dissolved in water remains. What are the products of this chemical reaction? ...
Metals, Nonmetals, and Metalloids (Vocabulary)
... Metals, Nonmetals, and Metalloids (Vocabulary) ...
... Metals, Nonmetals, and Metalloids (Vocabulary) ...
STRUCTURE_OF_THE_ATOM
... much changed for the next 1000 years. The ideas of Aristotle were still being taught. ...
... much changed for the next 1000 years. The ideas of Aristotle were still being taught. ...
atomic
... -larger number on periodic table -must be rounded to a whole number # of n0 = mass # - atomic # -element can be written as He-4 ...
... -larger number on periodic table -must be rounded to a whole number # of n0 = mass # - atomic # -element can be written as He-4 ...
Practice questions
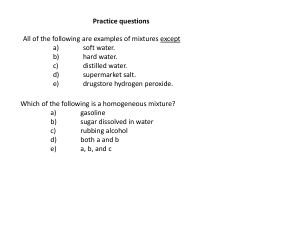
... Maleic acid contains 41.4% carbon, 3.47% hydrogen, and 55.1% oxygen by mass. A 0.050-mol sample of this compound weighs 5.80 g. What is the molecular formula of ...
... Maleic acid contains 41.4% carbon, 3.47% hydrogen, and 55.1% oxygen by mass. A 0.050-mol sample of this compound weighs 5.80 g. What is the molecular formula of ...
Notes
... In a ___________________ atom, the number of ___________________ will equal the number of _________________________ – meaning there will be NO overall ___________________ on the atom ...
... In a ___________________ atom, the number of ___________________ will equal the number of _________________________ – meaning there will be NO overall ___________________ on the atom ...
No Slide Title
... – Proton - a positively charged particle – Neutron - a neutral particle – Electron - a negatively charged particle (much lighter than a Proton or Neutron) ...
... – Proton - a positively charged particle – Neutron - a neutral particle – Electron - a negatively charged particle (much lighter than a Proton or Neutron) ...
Parts of the Atom - Issaquah Connect
... Parts of the Atom: 2 parts: Nucleus and electron cloud ________ ...
... Parts of the Atom: 2 parts: Nucleus and electron cloud ________ ...
Extra Credit Test Review
... 1. There are more than 110 known elements on the Periodic Table, but no element with an atomic number greater than 92 is found naturally in measurable quantities on Earth. The remaining elements are artificially produced in a laboratory setting. 2. What did Rutherford contribute to the Atomic Theory ...
... 1. There are more than 110 known elements on the Periodic Table, but no element with an atomic number greater than 92 is found naturally in measurable quantities on Earth. The remaining elements are artificially produced in a laboratory setting. 2. What did Rutherford contribute to the Atomic Theory ...
The History of the Atom - Brookville Local Schools
... X is the atomic symbol. If you match the number of protons to the atomic number on the periodic table, you can figure out what the symbol is supposed to be. ...
... X is the atomic symbol. If you match the number of protons to the atomic number on the periodic table, you can figure out what the symbol is supposed to be. ...
Atomic Structure
... •It is useful to compare the relative masses of atoms to a standard reference isotope. Carbon-12 is the standard reference isotope. Cabon12 has a mass of exactly 12 atomic mass units. •An atomic mass unit (amu) is defined as one twelfth of the mass of a carbon-12 atom. ...
... •It is useful to compare the relative masses of atoms to a standard reference isotope. Carbon-12 is the standard reference isotope. Cabon12 has a mass of exactly 12 atomic mass units. •An atomic mass unit (amu) is defined as one twelfth of the mass of a carbon-12 atom. ...
Chapter 3 STUDY GUIDE True/False Indicate whether the statement
... 13. Water and hydrogen peroxide have different properties because ____. a. they are made from different elements b. one contains a greater percentage of oxygen than the other c. one is a compound and one is a mixture d. only water follows the law of definite proportions ...
... 13. Water and hydrogen peroxide have different properties because ____. a. they are made from different elements b. one contains a greater percentage of oxygen than the other c. one is a compound and one is a mixture d. only water follows the law of definite proportions ...
Atoms, Molecules and Ions Part 2
... Isotopes • Dalton, had proposed that all atoms of the same element must have the same mass. • One of the 2 flaws in Dalton’s theory was, atoms of the same element can have DIFFERENT masses. • The mass of an atom is due to the mass of the protons and the neutrons in that atom. • Isotopes are atoms o ...
... Isotopes • Dalton, had proposed that all atoms of the same element must have the same mass. • One of the 2 flaws in Dalton’s theory was, atoms of the same element can have DIFFERENT masses. • The mass of an atom is due to the mass of the protons and the neutrons in that atom. • Isotopes are atoms o ...
Chap 03A-Atoms and Elements.pptx
... All the atoms of an element are identical to one another, but different from others. ...
... All the atoms of an element are identical to one another, but different from others. ...
Name: Date: Period: Page # Evolution of Atomic Theory (Changed
... As more evidence was collected over time, the theory and models were ________ ...
... As more evidence was collected over time, the theory and models were ________ ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.