Chapter 0 A Very Brief History of Chemistry Multiple Choice Questions
... elements can be generated depending on the masses used. e. When two different elements combine to form a mixture, they do so in definite proportions by weight. Answer: c Section 0.4 Difficulty Level: medium ...
... elements can be generated depending on the masses used. e. When two different elements combine to form a mixture, they do so in definite proportions by weight. Answer: c Section 0.4 Difficulty Level: medium ...
Chapter 12
... It is important to understand that when we say that the atomic mass of carbon is 12.01 amu, we are referring to the average value. If carbon atoms could be examined individually, we would find either an atom of atomic mass 12.00000 amu or one of 13.00335 amu, but never one of 12.01 amu. Example 3.1 ...
... It is important to understand that when we say that the atomic mass of carbon is 12.01 amu, we are referring to the average value. If carbon atoms could be examined individually, we would find either an atom of atomic mass 12.00000 amu or one of 13.00335 amu, but never one of 12.01 amu. Example 3.1 ...
Chapter 12
... It is important to understand that when we say that the atomic mass of carbon is 12.01 amu, we are referring to the average value. If carbon atoms could be examined individually, we would find either an atom of atomic mass 12.00000 amu or one of 13.00335 amu, but never one of 12.01 amu. Example 3.1 ...
... It is important to understand that when we say that the atomic mass of carbon is 12.01 amu, we are referring to the average value. If carbon atoms could be examined individually, we would find either an atom of atomic mass 12.00000 amu or one of 13.00335 amu, but never one of 12.01 amu. Example 3.1 ...
formula
... Each unit is preceded by a list of learning targets. Pay attention to these targets! Not every learning target has problems that go with it. Read the textbook! Make sure that after you complete a unit that you feel confident that you have mastered each target. It benefits you to take this assignment ...
... Each unit is preceded by a list of learning targets. Pay attention to these targets! Not every learning target has problems that go with it. Read the textbook! Make sure that after you complete a unit that you feel confident that you have mastered each target. It benefits you to take this assignment ...
chapter4
... • Some of these particles are called protons – charge = +1 – mass is about the same as a hydrogen atom • Since protons and electrons have the same amount of charge, for the atom to be neutral there must be equal numbers of protons and electrons • The other particle is called a neutron – has no charg ...
... • Some of these particles are called protons – charge = +1 – mass is about the same as a hydrogen atom • Since protons and electrons have the same amount of charge, for the atom to be neutral there must be equal numbers of protons and electrons • The other particle is called a neutron – has no charg ...
Dalton`s Atomic Theory
... • All substances are made of atoms. Atoms are the smallest particles of matter. They cannot be divided into smaller particles, created, or destroyed. • All atoms of the same element are alike and have the same mass. Atoms of different elements are different and have different masses. • Atoms join to ...
... • All substances are made of atoms. Atoms are the smallest particles of matter. They cannot be divided into smaller particles, created, or destroyed. • All atoms of the same element are alike and have the same mass. Atoms of different elements are different and have different masses. • Atoms join to ...
Chem 151 Chapter 2a
... • The spontaneous emission of radiation by an atom. • First observed by Henri Becquerel. • Also studied by Marie and Pierre Curie. ...
... • The spontaneous emission of radiation by an atom. • First observed by Henri Becquerel. • Also studied by Marie and Pierre Curie. ...
Worlds Within Worlds. The Story of Nuclear Energy Vol I
... were different from the atoms of every other element. The chief difference between the various atoms lay in their mass, or weight. Dalton was the first to try to determine what these masses might be. He could not work out the actual masses in ounces or grams, for atoms were f ...
... were different from the atoms of every other element. The chief difference between the various atoms lay in their mass, or weight. Dalton was the first to try to determine what these masses might be. He could not work out the actual masses in ounces or grams, for atoms were f ...
chapter 2 (w)
... Monatomic cations are named after the element. For example, Al3+ is called the aluminum ion. n If there is more than one cation of an element, a Roman numeral in parentheses denoting the charge on the ion is used. This often occurs with transition elements. n The names of the monatomic anions use th ...
... Monatomic cations are named after the element. For example, Al3+ is called the aluminum ion. n If there is more than one cation of an element, a Roman numeral in parentheses denoting the charge on the ion is used. This often occurs with transition elements. n The names of the monatomic anions use th ...
Practice Packet Level 3: Atomics - Mr. Palermo`s Flipped Chemistry
... field. This suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson concluded that the atom was a positively charged sphere of almost uniform density in which ...
... field. This suggested that cathode rays were composed of negatively charged particles found in all atoms. Thomson concluded that the atom was a positively charged sphere of almost uniform density in which ...
MidtermReview2012
... 2. How many valence electrons are present in a neutral atom of oxygen? 3. How many valence electrons are present in a neutral atom of lead? 4. For each of the following elements, write the number of valence electrons that a neutral atom of that element will have. a. Sodium b. Barium c. Fluorine d. A ...
... 2. How many valence electrons are present in a neutral atom of oxygen? 3. How many valence electrons are present in a neutral atom of lead? 4. For each of the following elements, write the number of valence electrons that a neutral atom of that element will have. a. Sodium b. Barium c. Fluorine d. A ...
TOPIC 12. THE ELEMENTS
... universe but is probably a second or third generation star, formed in part from the energy and residues released by previous supernovae. Although already 5 billion years old, it still contains 71% hydrogen and 27% helium, so the sun will burn for several billion more years. Thus on the basis of this ...
... universe but is probably a second or third generation star, formed in part from the energy and residues released by previous supernovae. Although already 5 billion years old, it still contains 71% hydrogen and 27% helium, so the sun will burn for several billion more years. Thus on the basis of this ...
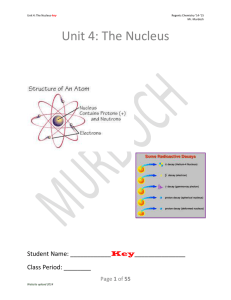
Unit 4: The Nucleus
... approximate mass of either a proton or neutron. 3. Atomic Number: The number that identifies an element, equal to an atom’s number of protons. 4. Deflect: Change in direction due to an outside force. 5. Emit: To give off something. 6. Half-life: The time it takes for half the mass of a radioactive i ...
... approximate mass of either a proton or neutron. 3. Atomic Number: The number that identifies an element, equal to an atom’s number of protons. 4. Deflect: Change in direction due to an outside force. 5. Emit: To give off something. 6. Half-life: The time it takes for half the mass of a radioactive i ...
Reduction and Emergence in Chemistry
... elements or the properties that a compound might have once any two or more elements have combined together. Moreover, it is not as though there was a complete absence of any theoretical understanding of chemical bonding before the quantum theory was introduced. Lewis’s theory, whereby covalent bonds ...
... elements or the properties that a compound might have once any two or more elements have combined together. Moreover, it is not as though there was a complete absence of any theoretical understanding of chemical bonding before the quantum theory was introduced. Lewis’s theory, whereby covalent bonds ...
Reduction and Emergence in Chemistry - Philsci
... elements or the properties that a compound might have once any two or more elements have combined together. Moreover, it is not as though there was a complete absence of any theoretical understanding of chemical bonding before the quantum theory was introduced. Lewis’s theory, whereby covalent bonds ...
... elements or the properties that a compound might have once any two or more elements have combined together. Moreover, it is not as though there was a complete absence of any theoretical understanding of chemical bonding before the quantum theory was introduced. Lewis’s theory, whereby covalent bonds ...
Chapter 14
... It is important to know the mass of the atoms especially for the lab work. However; atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it ...
... It is important to know the mass of the atoms especially for the lab work. However; atoms are very very small particles and we can not count it or weight it easily that because it contains huge number of atoms. For example the smallest thing we can see by our nicked eyes contains about 1016 atom, it ...
Chapter 7
... • K + Br2 → KBr • K + Br2 → 2KBr • First I add a 2 to the product to end with 2 bromine atoms, but now I am also ending with 2 potassium atoms. • 2K + Br2 → 2KBr • Adding a 2 in front of potassium fixes this. ...
... • K + Br2 → KBr • K + Br2 → 2KBr • First I add a 2 to the product to end with 2 bromine atoms, but now I am also ending with 2 potassium atoms. • 2K + Br2 → 2KBr • Adding a 2 in front of potassium fixes this. ...
GOOD NOTES CH3
... 1. All matter is composed of extremely small particles. 2. Atoms of the same element are chemically alike. Atoms of different elements are chemically different. 3. Atoms cannot be divided, created, nor destroyed. 4. Atoms combine in whole # ratios to form compounds. 5. Atoms are combined, separated, ...
... 1. All matter is composed of extremely small particles. 2. Atoms of the same element are chemically alike. Atoms of different elements are chemically different. 3. Atoms cannot be divided, created, nor destroyed. 4. Atoms combine in whole # ratios to form compounds. 5. Atoms are combined, separated, ...
Chapter 3
... A proton has a positive charge equal in magnitude to the negative charge of an electron. Atoms are electrically neutral because they contain equal numbers of protons and electrons. A neutron is electrically neutral. The nuclei of atoms of different elements differ in their number of protons and ther ...
... A proton has a positive charge equal in magnitude to the negative charge of an electron. Atoms are electrically neutral because they contain equal numbers of protons and electrons. A neutron is electrically neutral. The nuclei of atoms of different elements differ in their number of protons and ther ...
a. Matter First Day of Class
... Major Goals of Chapter 1: 1. Define the term chemistry. 2. Identify substances (matter) as chemicals. 3. Describe some physical and chemical properties of matter. 4. Describe the activities that are part of the scientific method. 5. Describe how you tell call whether you have a pure element or a com ...
... Major Goals of Chapter 1: 1. Define the term chemistry. 2. Identify substances (matter) as chemicals. 3. Describe some physical and chemical properties of matter. 4. Describe the activities that are part of the scientific method. 5. Describe how you tell call whether you have a pure element or a com ...
Topic 4 Chemistry of the Elements of the Main Group
... Hydrogen forms ionic hydrides with the reactive s-block metals (groups 1 and 2) and forms covalent hydrides with the p-group metals, e.g. Al and Sn (group 13 and 14). Electronegativity = 2.1. The value is intermediate in the electronegativity scale that spans from 0.7 to 4.0. H can form hydrides ( ...
... Hydrogen forms ionic hydrides with the reactive s-block metals (groups 1 and 2) and forms covalent hydrides with the p-group metals, e.g. Al and Sn (group 13 and 14). Electronegativity = 2.1. The value is intermediate in the electronegativity scale that spans from 0.7 to 4.0. H can form hydrides ( ...
Chapter 1 and Sections 3.1-3.3
... Chapter 1 and Sections 3.1-3.3 Major Goals of Chapter 1: 1. Define the term chemistry. 2. Identify substances (matter) as chemicals. 3. Describe some physical and chemical properties of matter. 4. Describe the activities that are part of the scientific method. 5. Describe how you tell call whether y ...
... Chapter 1 and Sections 3.1-3.3 Major Goals of Chapter 1: 1. Define the term chemistry. 2. Identify substances (matter) as chemicals. 3. Describe some physical and chemical properties of matter. 4. Describe the activities that are part of the scientific method. 5. Describe how you tell call whether y ...
2.ATOMS, MOLECULES, AND IONS
... they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two elements with the correct ratio of atoms, 1:2; therefore, it must be CO2. ...
... they don't contain the correct number of atoms. Keeping in mind that balls of the same color represent the same element, only the model on the far right contains two elements with the correct ratio of atoms, 1:2; therefore, it must be CO2. ...
Chemical Bonds - Warren County Public Schools
... Living organisms are composed of about 25 chemical elements • Living organisms are composed of matter, which is anything that occupies space and has mass (weight) – Matter is composed of chemical ...
... Living organisms are composed of about 25 chemical elements • Living organisms are composed of matter, which is anything that occupies space and has mass (weight) – Matter is composed of chemical ...
Chemical element
A chemical element (or element) is a chemical substance consisting of atoms having the same number of protons in their atomic nuclei (i.e. the same atomic number, Z). There are 118 elements that have been identified, of which the first 94 occur naturally on Earth with the remaining 24 being synthetic elements. There are 80 elements that have at least one stable isotope and 38 that have exclusively radioactive isotopes, which decay over time into other elements. Iron is the most abundant element (by mass) making up the Earth, while oxygen is the most common element in the crust of the earth.Chemical elements constitute approximately 15% of the matter in the universe: the remainder is dark matter, the composition of it is unknown, but it is not composed of chemical elements.The two lightest elements, hydrogen and helium were mostly formed in the Big Bang and are the most common elements in the universe. The next three elements (lithium, beryllium and boron) were formed mostly by cosmic ray spallation, and are thus more rare than those that follow. Formation of elements with from six to twenty six protons occurred and continues to occur in main sequence stars via stellar nucleosynthesis. The high abundance of oxygen, silicon, and iron on Earth reflects their common production in such stars. Elements with greater than twenty six protons are formed by supernova nucleosynthesis in supernovae, which, when they explode, blast these elements far into space as planetary nebulae, where they may become incorporated into planets when they are formed.When different elements are chemically combined, with the atoms held together by chemical bonds, they form chemical compounds. Only a minority of elements are found uncombined as relatively pure minerals. Among the more common of such ""native elements"" are copper, silver, gold, carbon (as coal, graphite, or diamonds), and sulfur. All but a few of the most inert elements, such as noble gases and noble metals, are usually found on Earth in chemically combined form, as chemical compounds. While about 32 of the chemical elements occur on Earth in native uncombined forms, most of these occur as mixtures. For example, atmospheric air is primarily a mixture of nitrogen, oxygen, and argon, and native solid elements occur in alloys, such as that of iron and nickel.The history of the discovery and use of the elements began with primitive human societies that found native elements like carbon, sulfur, copper and gold. Later civilizations extracted elemental copper, tin, lead and iron from their ores by smelting, using charcoal. Alchemists and chemists subsequently identified many more, with almost all of the naturally-occurring elements becoming known by 1900. The properties of the chemical elements are summarized on the periodic table, which organizes the elements by increasing atomic number into rows (""periods"") in which the columns (""groups"") share recurring (""periodic"") physical and chemical properties. Save for unstable radioactive elements with short half-lives, all of the elements are available industrially, most of them in high degrees of purity.