1.18 Cellular Respiration
... humans, cows, and fish? Which form is adequate for single-celled organisms such as yeast cells and bacteria? Explain. 3. Describe three cell processes that require the use of ATP. 4. (a) What is meant by the term exergonic reaction? (b) Is the formation of ATP during the reactions of glycolysis an e ...
... humans, cows, and fish? Which form is adequate for single-celled organisms such as yeast cells and bacteria? Explain. 3. Describe three cell processes that require the use of ATP. 4. (a) What is meant by the term exergonic reaction? (b) Is the formation of ATP during the reactions of glycolysis an e ...
Chapter 2 Chemistry Comes Alive
... The mass number of an atom is the sum of the masses of its protons and neutrons. The mass of the electrons is so small that it is ignored. Recall that protons and neutrons have a mass of 1 amu. Hydrogen has only one proton in its nucleus, so its atomic and mass numbers are the same: 1. Helium, with ...
... The mass number of an atom is the sum of the masses of its protons and neutrons. The mass of the electrons is so small that it is ignored. Recall that protons and neutrons have a mass of 1 amu. Hydrogen has only one proton in its nucleus, so its atomic and mass numbers are the same: 1. Helium, with ...
Reactions hydroxyl groups part-I
... Other possible ways Nucleophilic displacement of leaving groups, e.g. OMs or OTs, with hydride from reducing agents such as LiAlH4 is not generally a good route Nucleophilic subs,tu,on is much slower in ...
... Other possible ways Nucleophilic displacement of leaving groups, e.g. OMs or OTs, with hydride from reducing agents such as LiAlH4 is not generally a good route Nucleophilic subs,tu,on is much slower in ...
Understanding the balanced diet Learn the truth about sugar
... The Evolution of the Western Diet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27 The Meaning of Nutrient Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 Proteins, Carbohydrates, and Fats—Telling ...
... The Evolution of the Western Diet . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . 27 The Meaning of Nutrient Requirements . . . . . . . . . . . . . . . . . . . . . . . . . . . . 28 Proteins, Carbohydrates, and Fats—Telling ...
Treatment
... Glyc/o/protein, Gluc/o/protein: substance made of sugar and protein Glycos/uria, Glucos/uria: Sugar in the urine Glyc/o/hem/o/globin: Sugar and hemoglobin (sugar attached to hemoglobin. Used to evaluate sugar level control in diabetic patients.) ...
... Glyc/o/protein, Gluc/o/protein: substance made of sugar and protein Glycos/uria, Glucos/uria: Sugar in the urine Glyc/o/hem/o/globin: Sugar and hemoglobin (sugar attached to hemoglobin. Used to evaluate sugar level control in diabetic patients.) ...
Analysis of Elements and Foods for the Human Body and
... of water in the body. Chlorine (0.15%) is usually found in the body as a negative ion, called chloride. This electrolyte is important for maintaining a normal balance of fluids. Iron (0.006%) is a key element in the metabolism of almost all living organisms. It is also found in haemoglobin, which is ...
... of water in the body. Chlorine (0.15%) is usually found in the body as a negative ion, called chloride. This electrolyte is important for maintaining a normal balance of fluids. Iron (0.006%) is a key element in the metabolism of almost all living organisms. It is also found in haemoglobin, which is ...
Exercise Physiology
... - By heavy training/ eating a diet rich is protein/ low in carbohydrates - 3 -4 days before competition training is reduced and performer eats a diet rich is carbohydrate - The body compensates for its previous lack of carbohydrate - Storing more glycogen than before/ increasing glycogen levels - Be ...
... - By heavy training/ eating a diet rich is protein/ low in carbohydrates - 3 -4 days before competition training is reduced and performer eats a diet rich is carbohydrate - The body compensates for its previous lack of carbohydrate - Storing more glycogen than before/ increasing glycogen levels - Be ...
Document
... building of new breaking down of complex substances. substances. • The destructive AKA- Synthesis phase of your metabolism AKA: (Digestion or Hydrolysis) ...
... building of new breaking down of complex substances. substances. • The destructive AKA- Synthesis phase of your metabolism AKA: (Digestion or Hydrolysis) ...
Hein and Arena - faculty at Chemeketa
... With formaldehyde, primary alcohols are formed; with other aldehydes, secondary alcohols are formed; with ketones, tertiary alcohols are formed. ...
... With formaldehyde, primary alcohols are formed; with other aldehydes, secondary alcohols are formed; with ketones, tertiary alcohols are formed. ...
Biological Classification of Mustard Plant
... salivary glands in response to the presence of food in the buccal cavity. Saliva is alkaline and contains an enzyme ptyalin. This enzyme converts starch into sugar (maltose). The morsel of food after being chewed and thoroughly mixed with the saliva is called a bolus. It is rolled down by the swallo ...
... salivary glands in response to the presence of food in the buccal cavity. Saliva is alkaline and contains an enzyme ptyalin. This enzyme converts starch into sugar (maltose). The morsel of food after being chewed and thoroughly mixed with the saliva is called a bolus. It is rolled down by the swallo ...
Bio 20 A - Holy Trinity Academy
... that interact with other polar molecules such as water, and with nonpolar regions where differences in saturation determine the structure and function of lipids. 4. Carbohydrates are composed of sugar monomers whose structures and bonding with each other by dehydration synthesis determine the proper ...
... that interact with other polar molecules such as water, and with nonpolar regions where differences in saturation determine the structure and function of lipids. 4. Carbohydrates are composed of sugar monomers whose structures and bonding with each other by dehydration synthesis determine the proper ...
1 category question correct answer your answer
... Glycogen metabolism increases the glucose levels within cells, while decreasing glucose levels in blood. Insulin stimulates uptake of glucose from the bloodstream into cells and ...
... Glycogen metabolism increases the glucose levels within cells, while decreasing glucose levels in blood. Insulin stimulates uptake of glucose from the bloodstream into cells and ...
Preview Sample 1
... A. They form enzymes to speed up reactions. B. They form the backbone of cell membranes. C. They form body parts such as muscle. D. They form antibodies to protect the body from disease. Phospholipids form the backbone of cell membranes. ...
... A. They form enzymes to speed up reactions. B. They form the backbone of cell membranes. C. They form body parts such as muscle. D. They form antibodies to protect the body from disease. Phospholipids form the backbone of cell membranes. ...
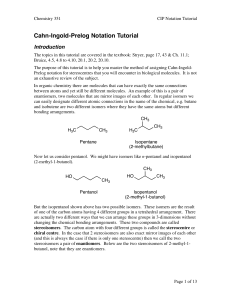
Cahn-Ingold-Prelog Notation Tutorial
... But the isopentanol shown above has two possible isomers. These isomers are the result of one of the carbon atoms having 4 different groups in a tetrahedral arrangement. There are actually two different ways that we can arrange these groups in 3-dimensions without changing the chemical bonding arran ...
... But the isopentanol shown above has two possible isomers. These isomers are the result of one of the carbon atoms having 4 different groups in a tetrahedral arrangement. There are actually two different ways that we can arrange these groups in 3-dimensions without changing the chemical bonding arran ...
Document
... 1 a large surface area for this swapping of gases to take place 2 ways to prevent the surfaces being clogged up with dust from the air 3 a good blood supply and circulatory system to move things around the body. How many times do you breathe in a minute? © OUP: To be used solely in purchaser’s schoo ...
... 1 a large surface area for this swapping of gases to take place 2 ways to prevent the surfaces being clogged up with dust from the air 3 a good blood supply and circulatory system to move things around the body. How many times do you breathe in a minute? © OUP: To be used solely in purchaser’s schoo ...
aldehyde group - Imperial Valley College Faculty Websites
... Some aldehydes have common names as seen this table. ...
... Some aldehydes have common names as seen this table. ...
Chemical Basis of Life - SBCC Biological Sciences Department
... chloride ions react to form a type of chemical bond called an ionic bond (electrovalent bond). Sodium ions (Na+) and chloride ions (Cl–) uniting in this manner form the compound sodium chloride (NaCl), or table salt (fig. 2.4b). Some ions have an electrical charge greater than 1—for example, Ca+2 (o ...
... chloride ions react to form a type of chemical bond called an ionic bond (electrovalent bond). Sodium ions (Na+) and chloride ions (Cl–) uniting in this manner form the compound sodium chloride (NaCl), or table salt (fig. 2.4b). Some ions have an electrical charge greater than 1—for example, Ca+2 (o ...
FREE Sample Here
... 28. (p. 24) Which of the following is NOT a function of proteins? A. They form enzymes to speed up reactions. B. They form the backbone of cell membranes. C. They form body parts such as muscle. D. They form antibodies to protect the body from disease. Phospholipids form the backbone of cell membran ...
... 28. (p. 24) Which of the following is NOT a function of proteins? A. They form enzymes to speed up reactions. B. They form the backbone of cell membranes. C. They form body parts such as muscle. D. They form antibodies to protect the body from disease. Phospholipids form the backbone of cell membran ...
FREE Sample Here
... 28. (p. 24) Which of the following is NOT a function of proteins? A. They form enzymes to speed up reactions. B. They form the backbone of cell membranes. C. They form body parts such as muscle. D. They form antibodies to protect the body from disease. Phospholipids form the backbone of cell membran ...
... 28. (p. 24) Which of the following is NOT a function of proteins? A. They form enzymes to speed up reactions. B. They form the backbone of cell membranes. C. They form body parts such as muscle. D. They form antibodies to protect the body from disease. Phospholipids form the backbone of cell membran ...
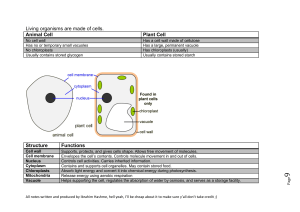
Living organisms are made of cells. Animal Cell Plant Cell Structure
... Plant must have chlorophyll in order to photosynthesise, as this is the substance which converts light energy into chemical energy. Chlorophyll traps light energy and converts it into chemical energy for the formation of carbohydrates and their subsequent storage. Therefore, it goes without saying, ...
... Plant must have chlorophyll in order to photosynthesise, as this is the substance which converts light energy into chemical energy. Chlorophyll traps light energy and converts it into chemical energy for the formation of carbohydrates and their subsequent storage. Therefore, it goes without saying, ...
Bio 20 Reg - Holy Trinity Academy
... fats together. This is called hydrolysis. This involves the addition of a water molecule into the carbohydrate, protein or fat splitting the compound into two smaller compounds. The opposite process, the building of a larger molecule from two smaller molecules by removing an H atom and an OH group f ...
... fats together. This is called hydrolysis. This involves the addition of a water molecule into the carbohydrate, protein or fat splitting the compound into two smaller compounds. The opposite process, the building of a larger molecule from two smaller molecules by removing an H atom and an OH group f ...
$doc.title
... Uses of the Wing Reac3on • Can be used for monosubs3tuted, disubs3tuted, and trisubs3tuted alkenes but not tetrasubs3tuted alkenes The reac3on yields a pure alkene of known structure • For comparison, addi3 ...
... Uses of the Wing Reac3on • Can be used for monosubs3tuted, disubs3tuted, and trisubs3tuted alkenes but not tetrasubs3tuted alkenes The reac3on yields a pure alkene of known structure • For comparison, addi3 ...
Macromolecules in Biological System.doc
... c) – Existence of molecules with the same composition but different structures 10. Asymmetrical carbon is: b) – Carbon with four different bonds 11. Glucose is: a) – Aldose 12. Fructose is: b) – Ketose 13. In sugars the carbon atom that determines the series is: b) – The farthest from the main funct ...
... c) – Existence of molecules with the same composition but different structures 10. Asymmetrical carbon is: b) – Carbon with four different bonds 11. Glucose is: a) – Aldose 12. Fructose is: b) – Ketose 13. In sugars the carbon atom that determines the series is: b) – The farthest from the main funct ...
Chapter 1 - Coastal Bend College
... A. Protection: surrounds and protects organs B. Insulation: fat under the skin prevents heat loss; myelin sheaths electrically insulate axons of neurons C. Regulation: steroids regulates physiological processes ...
... A. Protection: surrounds and protects organs B. Insulation: fat under the skin prevents heat loss; myelin sheaths electrically insulate axons of neurons C. Regulation: steroids regulates physiological processes ...
Carbohydrate
A carbohydrate is a biological molecule consisting of carbon (C), hydrogen (H) and oxygen (O) atoms, usually with a hydrogen:oxygen atom ratio of 2:1 (as in water); in other words, with the empirical formula Cm(H2O)n (where m could be different from n). Some exceptions exist; for example, deoxyribose, a sugar component of DNA, has the empirical formula C5H10O4. Carbohydrates are technically hydrates of carbon; structurally it is more accurate to view them as polyhydroxy aldehydes and ketones.The term is most common in biochemistry, where it is a synonym of saccharide, a group that includes sugars, starch, and cellulose. The saccharides are divided into four chemical groups: monosaccharides, disaccharides, oligosaccharides, and polysaccharides. In general, the monosaccharides and disaccharides, which are smaller (lower molecular weight) carbohydrates, are commonly referred to as sugars. The word saccharide comes from the Greek word σάκχαρον (sákkharon), meaning ""sugar."" While the scientific nomenclature of carbohydrates is complex, the names of the monosaccharides and disaccharides very often end in the suffix -ose. For example, grape sugar is the monosaccharide glucose, cane sugar is the disaccharide sucrose and milk sugar is the disaccharide lactose (see illustration).Carbohydrates perform numerous roles in living organisms. Polysaccharides serve for the storage of energy (e.g., starch and glycogen) and as structural components (e.g., cellulose in plants and chitin in arthropods). The 5-carbon monosaccharide ribose is an important component of coenzymes (e.g., ATP, FAD and NAD) and the backbone of the genetic molecule known as RNA. The related deoxyribose is a component of DNA. Saccharides and their derivatives include many other important biomolecules that play key roles in the immune system, fertilization, preventing pathogenesis, blood clotting, and development.In food science and in many informal contexts, the term carbohydrate often means any food that is particularly rich in the complex carbohydrate starch (such as cereals, bread and pasta) or simple carbohydrates, such as sugar (found in candy, jams, and desserts).