Unit 3 Atomic Structure
... are represented with Nuclear Equations. • Transmutation reactions – a reaction where one nucleus changes element. (# of protons) ...
... are represented with Nuclear Equations. • Transmutation reactions – a reaction where one nucleus changes element. (# of protons) ...
Chapter 2 - profpaz.com
... Atoms of the same element (same atomic number) can possess different number of neutrons (different mass numbers) and are called isotopes. Most elements have several isotopes, which are indicated by its chemical symbol, followed by a dash and the mass number of isotope. For example, the 3 isotopes of ...
... Atoms of the same element (same atomic number) can possess different number of neutrons (different mass numbers) and are called isotopes. Most elements have several isotopes, which are indicated by its chemical symbol, followed by a dash and the mass number of isotope. For example, the 3 isotopes of ...
Chapter 2: Matter is Made up of Atoms
... Why e-s (usually) don’t fly off of atoms: they have enough attraction to the nucleus to keep them in “orbit.” ...
... Why e-s (usually) don’t fly off of atoms: they have enough attraction to the nucleus to keep them in “orbit.” ...
Regents questions
... Sample 7.1 Natural gas used in home heating and cooking is odorless. Because natural gas leaks pose the danger of explosion or suffocation, various smelly substances are added to the gas to allow detection of a leak. One such substance is methyl mercaptan, CH3SH. Use Figure 7.6 to predict the lengt ...
... Sample 7.1 Natural gas used in home heating and cooking is odorless. Because natural gas leaks pose the danger of explosion or suffocation, various smelly substances are added to the gas to allow detection of a leak. One such substance is methyl mercaptan, CH3SH. Use Figure 7.6 to predict the lengt ...
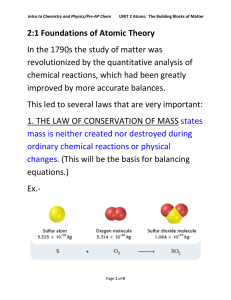
2:1 Foundations of Atomic Theory In the 1790s the study of matter
... neutron are approximately the same size and are 2000 times larger than an electron. If a large football stadium were an atom, this would make the nucleus (including the protons and neutrons) about the size of a marble. The electrons would be similar to dust particles floating about the stadium. This ...
... neutron are approximately the same size and are 2000 times larger than an electron. If a large football stadium were an atom, this would make the nucleus (including the protons and neutrons) about the size of a marble. The electrons would be similar to dust particles floating about the stadium. This ...
Chapter 4 Packet Chem
... Protons, electrons, and neutrons can be distinguished by ___________________, ___________________, and ___________________ in the atom. Atoms of different elements have different numbers of ___________________. Isotopes of an element have the same numbers of ___________________ but different numbers ...
... Protons, electrons, and neutrons can be distinguished by ___________________, ___________________, and ___________________ in the atom. Atoms of different elements have different numbers of ___________________. Isotopes of an element have the same numbers of ___________________ but different numbers ...
C1 - Powerpoint - tonyconnett.com
... 2. Group 1 atoms want to loose an electron, do you think it would be easier to remove an electron from Lithium or Cs? 3. What is the most reactive element in group 1? 4. Group 7 atoms want to gain an electron, which atom would most strongly attract an electron to the ‘gap’ in the outer shell? 5. Whi ...
... 2. Group 1 atoms want to loose an electron, do you think it would be easier to remove an electron from Lithium or Cs? 3. What is the most reactive element in group 1? 4. Group 7 atoms want to gain an electron, which atom would most strongly attract an electron to the ‘gap’ in the outer shell? 5. Whi ...
PowerPoint プレゼンテーション
... The octet rule says that atoms will be more stable when they have a full outer shell… usually 8 valence electrons. ...
... The octet rule says that atoms will be more stable when they have a full outer shell… usually 8 valence electrons. ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Just like members of the same family, they share similar characteristics. Each element family has a ...
... Just like members of the same family, they share similar characteristics. Each element family has a ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Just like members of the same family, they share similar characteristics. Each element family has a ...
... Just like members of the same family, they share similar characteristics. Each element family has a ...
chapter 4 - Elkhorn Valley Schools
... He thought they were indivisible and indestructible “atomists” – people who followed his beliefs ...
... He thought they were indivisible and indestructible “atomists” – people who followed his beliefs ...
Chemistry
... taking into account the percent and mass of each different isotope. C4.10e C4.10c Calculate the average atomic mass of an element given the percent abundance and mass of the individual isotopes. C4.10d Predict which isotope will have the greatest abundance given the possible isotopes for an element ...
... taking into account the percent and mass of each different isotope. C4.10e C4.10c Calculate the average atomic mass of an element given the percent abundance and mass of the individual isotopes. C4.10d Predict which isotope will have the greatest abundance given the possible isotopes for an element ...
Physical Science 1st Semester final Review
... 87.Moving from left to right across a row of the periodic table, what value increases by exactly one from element to element? ...
... 87.Moving from left to right across a row of the periodic table, what value increases by exactly one from element to element? ...
Chapter 4: Introduction to Earth Chemistry Section 1 Notes
... _____________ a group of atoms that are held together by chemical forces; a molecule is the smallest unit of matter that can exist by itself and retain all of a substance’s chemical properties Chemical Formulas A chemical formula is a _________________________________________________________________ ...
... _____________ a group of atoms that are held together by chemical forces; a molecule is the smallest unit of matter that can exist by itself and retain all of a substance’s chemical properties Chemical Formulas A chemical formula is a _________________________________________________________________ ...
Chapter Three: Atoms and Atomic Masses
... We symbolize this ion as Al3+. Note that losing electrons is indicated with +, and gaining electrons is indicated with -. ...
... We symbolize this ion as Al3+. Note that losing electrons is indicated with +, and gaining electrons is indicated with -. ...
Atomic Structure - OCPS TeacherPress
... The number of valence electrons in an atom will determine if an element will allow electricity to flow. The ability of an atom to draw electrons to itself (away from its neighbors) is called Electronegativity. ...
... The number of valence electrons in an atom will determine if an element will allow electricity to flow. The ability of an atom to draw electrons to itself (away from its neighbors) is called Electronegativity. ...
Atoms Ions Valence Electrons Isotopes
... valence electrons of an atom of a particular element are shown, indicated by dots placed around the element’s symbol ▪ also called electron-dot notation ▪ an electron is placed on each of the four “sides” of the element symbol before a second electron is added to any side ...
... valence electrons of an atom of a particular element are shown, indicated by dots placed around the element’s symbol ▪ also called electron-dot notation ▪ an electron is placed on each of the four “sides” of the element symbol before a second electron is added to any side ...
Chapter 2.1, 2.2 Review Packet – Answer Key
... Atoms of the same element that differ in the number of neutrons are called isotopes. Isotopes are identified by their mass number, the total number of protons and neutrons in the nucleus. Because they have the same number of electrons in each atom, all isotopes of an element have the same chemical p ...
... Atoms of the same element that differ in the number of neutrons are called isotopes. Isotopes are identified by their mass number, the total number of protons and neutrons in the nucleus. Because they have the same number of electrons in each atom, all isotopes of an element have the same chemical p ...
Chapter 5 Chem classnotes
... As atomic numbers get bigger (one per element), this means that each subsequent element has one more ___________. Because of this increasing nuclear charge (or positive charge), the electrons orbiting the nucleus are pulled in closer and closer. ...
... As atomic numbers get bigger (one per element), this means that each subsequent element has one more ___________. Because of this increasing nuclear charge (or positive charge), the electrons orbiting the nucleus are pulled in closer and closer. ...
Ch 3: Atoms
... 3. Atoms can not be broken down, created or destroyed. (NOT TRUE) 4. Atoms combine in simple whole number ratios to form chemical compounds 5. A chemical reaction is the combining, separation, or rearrangement of atoms. ...
... 3. Atoms can not be broken down, created or destroyed. (NOT TRUE) 4. Atoms combine in simple whole number ratios to form chemical compounds 5. A chemical reaction is the combining, separation, or rearrangement of atoms. ...
SNC 1D Chemistry Review
... 5. Isotopes of an element have: a) The same number of protons and neutrons b) The same number of protons, but a different number of electrons c) The same number of protons and electrons, but a different number of neutrons d) The same number of neutrons, but a different number of protons 6. What is ...
... 5. Isotopes of an element have: a) The same number of protons and neutrons b) The same number of protons, but a different number of electrons c) The same number of protons and electrons, but a different number of neutrons d) The same number of neutrons, but a different number of protons 6. What is ...
Chapter 5
... Beta Decay The emission of an electron from the nucleus and the transformation of the atom into a different element with the next higher atomic # is the result. ...
... Beta Decay The emission of an electron from the nucleus and the transformation of the atom into a different element with the next higher atomic # is the result. ...
Chapter 04
... electrons indicated by the symbol. You should also be able to use the number of protons, neutrons, and electrons to determine the corresponding atomic symbol. What are ions? Isotopes? You should be able to recognize if two atomic symbols are ions or isotopes of each other. What is the dif ...
... electrons indicated by the symbol. You should also be able to use the number of protons, neutrons, and electrons to determine the corresponding atomic symbol. What are ions? Isotopes? You should be able to recognize if two atomic symbols are ions or isotopes of each other. What is the dif ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Just like members of the same family, they share similar characteristics. Each element family has a ...
... Just like members of the same family, they share similar characteristics. Each element family has a ...
Atoms, Elements, and the Periodic Table Part 1: The Atomic Model
... Just like members of the same family, they share similar characteristics. Each element family has a ...
... Just like members of the same family, they share similar characteristics. Each element family has a ...