Properties of Atoms and the Periodic Table
... increasing numbers of protons and electrons. One proton and one electron are added to each element as you go across the periodic table from left to right. ...
... increasing numbers of protons and electrons. One proton and one electron are added to each element as you go across the periodic table from left to right. ...
Periodic Table
... this concept because the valence electrons are the electrons involved in bonding. You determine the valence electrons by counting the "s" and "p" electrons in that period. You can determine that fluorine has seven valence electrons by going to the second period and count over seven times. How many b ...
... this concept because the valence electrons are the electrons involved in bonding. You determine the valence electrons by counting the "s" and "p" electrons in that period. You can determine that fluorine has seven valence electrons by going to the second period and count over seven times. How many b ...
SCIENCE: EIGHTH GRADE CRT FIRST QUARTER
... Which subatomic particles are found in the nucleus of an atom? Where are electrons located in an atom? Who was the Greek philosopher responsible for naming the atom? Which two subatomic particles have the greatest mass? An element that is a very reactive gas is most likely a member H of which group ...
... Which subatomic particles are found in the nucleus of an atom? Where are electrons located in an atom? Who was the Greek philosopher responsible for naming the atom? Which two subatomic particles have the greatest mass? An element that is a very reactive gas is most likely a member H of which group ...
SCIENCE: EIGHTH GRADE CRT FIRST QUARTER
... Which subatomic particles are found in the nucleus of an atom? Where are electrons located in an atom? Who was the Greek philosopher responsible for naming the atom? Which two subatomic particles have the greatest mass? An element that is a very reactive gas is most likely a member H of which group ...
... Which subatomic particles are found in the nucleus of an atom? Where are electrons located in an atom? Who was the Greek philosopher responsible for naming the atom? Which two subatomic particles have the greatest mass? An element that is a very reactive gas is most likely a member H of which group ...
SCIENCE: EIGHTH GRADE CRT FIRST QUARTER
... Which subatomic particles are found in the nucleus of an atom? Where are electrons located in an atom? Who was the Greek philosopher responsible for naming the atom? Which two subatomic particles have the greatest mass? An element that is a very reactive gas is most likely a member H of which group ...
... Which subatomic particles are found in the nucleus of an atom? Where are electrons located in an atom? Who was the Greek philosopher responsible for naming the atom? Which two subatomic particles have the greatest mass? An element that is a very reactive gas is most likely a member H of which group ...
投影片 - 中正大學化生系
... 3. The arrangement of the elements in groups of elements in the order of their atomic weights corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of th ...
... 3. The arrangement of the elements in groups of elements in the order of their atomic weights corresponds to their so-called valencies, as well as, to some extent, to their distinctive chemical properties; as is apparent among other series in that of Li, Be, B, C, N, O, and F. 4. The magnitude of th ...
3-4 Bohr and Lewis
... Follow the 2, 8, 8 , 8, 8, 8….Rule to determine if the element is Happy or Unhappy (Stable or Unstable) An atom is always neutral. It has no net charge. Every carbon atom has 6 protons, it must have 6 electrons. Electrons in an atom are arranged in energy levels (or shells) around the nucleus. The e ...
... Follow the 2, 8, 8 , 8, 8, 8….Rule to determine if the element is Happy or Unhappy (Stable or Unstable) An atom is always neutral. It has no net charge. Every carbon atom has 6 protons, it must have 6 electrons. Electrons in an atom are arranged in energy levels (or shells) around the nucleus. The e ...
Using your periodic table (9/30-10/6) File
... orbitals & number of valence e-, & to know that properties are similar for elements in a group • Describe the structure of atoms, including the masses, electrical charges, and locations of protons, neutrons, and electrons • Identify that protons determine an elements identity and valence electrons d ...
... orbitals & number of valence e-, & to know that properties are similar for elements in a group • Describe the structure of atoms, including the masses, electrical charges, and locations of protons, neutrons, and electrons • Identify that protons determine an elements identity and valence electrons d ...
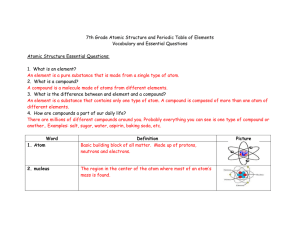
7th Grade Atomic Structure and Periodic Table of Elements
... There are millions of different compounds around you. Probably everything you can see is one type of compound or ...
... There are millions of different compounds around you. Probably everything you can see is one type of compound or ...
Introduction to the Atom
... The amu is the measurement of weight of an atom. The amu is the atom mass units. The amu system of measurement assigns one (1) amu to both protons and neutrons. The atomic number represents the number of protons an atom contains in the nucleus. An example is oxygen. The atomic number of oxygen is ...
... The amu is the measurement of weight of an atom. The amu is the atom mass units. The amu system of measurement assigns one (1) amu to both protons and neutrons. The atomic number represents the number of protons an atom contains in the nucleus. An example is oxygen. The atomic number of oxygen is ...
Midterm Review 2017
... 9) The diagram below represents the bright-line spectra of four elements and a bright-line spectrum produced by a mixture of three of these elements. ...
... 9) The diagram below represents the bright-line spectra of four elements and a bright-line spectrum produced by a mixture of three of these elements. ...
Lesson x- Review W14 answers
... pudding -discovered charge in the atom Rutherford -atoms are mostly empty space (where electrons are) with a dense positive centre (nucleus) -used the gold foil experiment to discover the nucleus; most particles went through, few bounced back Bohr -atoms have a dense nucleus surround by shells (ener ...
... pudding -discovered charge in the atom Rutherford -atoms are mostly empty space (where electrons are) with a dense positive centre (nucleus) -used the gold foil experiment to discover the nucleus; most particles went through, few bounced back Bohr -atoms have a dense nucleus surround by shells (ener ...
Unit III * Introduction to Atomic Theory
... The electrons orbit a large distance away from the nucleus. Proposes a “Solar System” or “Orbital” model of the atom Cute Summary of History ...
... The electrons orbit a large distance away from the nucleus. Proposes a “Solar System” or “Orbital” model of the atom Cute Summary of History ...
Basic Background Review: Acid-Base , Redox, and Stable Isotopes
... reactants (negative ΔH) contributes to spontaneous reaction) •ΔS measure of relative degree of disorder in product and reactant—greater disorder in product than reactant (positive ΔS) contributes to spontaneous reaction ...
... reactants (negative ΔH) contributes to spontaneous reaction) •ΔS measure of relative degree of disorder in product and reactant—greater disorder in product than reactant (positive ΔS) contributes to spontaneous reaction ...
Name: _key Date: ______ Period: Unit 3 – Atomic Structure Review
... 1. Who was the ancient Greek philosopher who first proposed the notion of the atom? Democritus 2. What was Dalton’s atomic model called? Billard Ball Model 3. Who’s model first introduced the concept of energy levels? Bohr 4. What were the major problems of Dalton’s atomic theory? He was wrong about ...
... 1. Who was the ancient Greek philosopher who first proposed the notion of the atom? Democritus 2. What was Dalton’s atomic model called? Billard Ball Model 3. Who’s model first introduced the concept of energy levels? Bohr 4. What were the major problems of Dalton’s atomic theory? He was wrong about ...
Atoms and the Periodic Table PowerPoint
... began working on his great achievement: the periodic table of the elements. By arranging all of the 63 elements then known by their atomic weights, he managed to organize them into groups possessing similar properties. Where a gap existed in the table, he predicted a new element would one day be fou ...
... began working on his great achievement: the periodic table of the elements. By arranging all of the 63 elements then known by their atomic weights, he managed to organize them into groups possessing similar properties. Where a gap existed in the table, he predicted a new element would one day be fou ...
The Periodic Table - Harlan Independent Schools
... The nitrogen family is named after the element that makes up 78% of our atmosphere. This family includes non-metals, metalloids, and metals. They tend to share electrons when they bond. Other elements in this family are phosphorus, arsenic, antimony, and bismuth. ...
... The nitrogen family is named after the element that makes up 78% of our atmosphere. This family includes non-metals, metalloids, and metals. They tend to share electrons when they bond. Other elements in this family are phosphorus, arsenic, antimony, and bismuth. ...
Chapter 5: The Periodic Law
... • These are the metals in Group 1 (1A): Li, Na, K, Rb, Cs, and Fr. The name for the family is derived from the Arabic word meaning “ashes” because we find Na and K in the ashes of burnt plants. • *Properties* These metals are: malleable, ductile and good conductors with low densities and low melting ...
... • These are the metals in Group 1 (1A): Li, Na, K, Rb, Cs, and Fr. The name for the family is derived from the Arabic word meaning “ashes” because we find Na and K in the ashes of burnt plants. • *Properties* These metals are: malleable, ductile and good conductors with low densities and low melting ...
Properties of Metals vs. Nonmetals vs. Metalloids
... Using the periodic table on page 10 of this study guide, answer the following questions: 1. Which element stands alone in its family? _______________ 2. Which element has a larger atomic radius O or Ca? _____________ 3. Which element has a larger atomic radius Ca or Ba? _____________ 4. Which elemen ...
... Using the periodic table on page 10 of this study guide, answer the following questions: 1. Which element stands alone in its family? _______________ 2. Which element has a larger atomic radius O or Ca? _____________ 3. Which element has a larger atomic radius Ca or Ba? _____________ 4. Which elemen ...
Atoms and the Periodic Table
... table by increasing atomic number. 1. In the late 1800’s, Dmitri Mendeleev devised the first periodic table based on atomic mass. 2. In 1913, Henry G.J. Moseley arranged the elements by atomic number rather than atomic mass. ...
... table by increasing atomic number. 1. In the late 1800’s, Dmitri Mendeleev devised the first periodic table based on atomic mass. 2. In 1913, Henry G.J. Moseley arranged the elements by atomic number rather than atomic mass. ...
Agenda/To Do - Perry Local Schools
... B. Non-metals- right side of periodic table (except H) C. Metalloids- form a zig zag line splitting the metals and non-metals ...
... B. Non-metals- right side of periodic table (except H) C. Metalloids- form a zig zag line splitting the metals and non-metals ...
GCSE Radiation - Bishopston Comprehensive School Moodle
... Each time a Uranium atom splits up it spits out 2 or 3 neutrons, one of which hits another uranium nucleus, causing it to split. This keeps the chain reaction going. ...
... Each time a Uranium atom splits up it spits out 2 or 3 neutrons, one of which hits another uranium nucleus, causing it to split. This keeps the chain reaction going. ...
Day 2 – Worksheet Atoms and The Periodic Table
... Atoms and The Periodic Table Worksheet 1. Define chemistry. ...
... Atoms and The Periodic Table Worksheet 1. Define chemistry. ...
ch3 B - Manasquan Public Schools
... Later on, the discovery of protons and neutrons were discovered in the nucleus. And it was later concluded that all atoms are neutral in charge. The number of protons and electrons in any atom are always equal. ...
... Later on, the discovery of protons and neutrons were discovered in the nucleus. And it was later concluded that all atoms are neutral in charge. The number of protons and electrons in any atom are always equal. ...