Honors Chemistry Chapter 4 Student Notes
... Technology and Society: In 1931, Ernst Ruska and Max Knoll built the first electron microscope. It uses an electron beam and “lenses” that consist of magnetic or electric fields. Objects can be magnified over 100,000 times and projected on to a monitor. Biochemistry, Microelectronics, and Biology a ...
... Technology and Society: In 1931, Ernst Ruska and Max Knoll built the first electron microscope. It uses an electron beam and “lenses” that consist of magnetic or electric fields. Objects can be magnified over 100,000 times and projected on to a monitor. Biochemistry, Microelectronics, and Biology a ...
Atomic - zsnedu
... identical. Atoms of any one element are different from those of any other element. ...
... identical. Atoms of any one element are different from those of any other element. ...
Dalton`s Atomic Theory
... number of atoms: 6.02 x 1023 atoms • Used to convert mass <-> moles <-> number of atoms or particles. • From the periodic chart, the atomic mass tells the number of grams in a mole of an element. ...
... number of atoms: 6.02 x 1023 atoms • Used to convert mass <-> moles <-> number of atoms or particles. • From the periodic chart, the atomic mass tells the number of grams in a mole of an element. ...
LAB ACTIVITY CALCULATING ATOMIC MASS
... believed to be element 114. The researchers have proposed that the element be named vabeachium (va-beach-e-m), after the city in which it was discovered. The proposed element symbol is Vb. Samples are currently being distributed to all of the local high schools. Each school is to isolate the individ ...
... believed to be element 114. The researchers have proposed that the element be named vabeachium (va-beach-e-m), after the city in which it was discovered. The proposed element symbol is Vb. Samples are currently being distributed to all of the local high schools. Each school is to isolate the individ ...
Chapter 3 - Vocabulary and Notes
... combine and whose properties are different from each of the elements in it (pg 87) Mixtures: A combination of compounds and elements that has not formed a new substance and whose proportions can be changed without changing the mixtures identity (pg 89) ...
... combine and whose properties are different from each of the elements in it (pg 87) Mixtures: A combination of compounds and elements that has not formed a new substance and whose proportions can be changed without changing the mixtures identity (pg 89) ...
Atomic Structure
... Weighted grades analogy Masses of subatomic particles ridiculously small – needed something more convenient Arbitrary amount set: Carbon-12 atom = 12.00000 amu 1 amu (atomic mass unit) = 1/12 mass of C-12 atom Mass of a single proton/neutron – 1 amu Why are the masses decimals? Most elements exist ...
... Weighted grades analogy Masses of subatomic particles ridiculously small – needed something more convenient Arbitrary amount set: Carbon-12 atom = 12.00000 amu 1 amu (atomic mass unit) = 1/12 mass of C-12 atom Mass of a single proton/neutron – 1 amu Why are the masses decimals? Most elements exist ...
Multiple choice questions
... Three atoms, I, II and III, each have an atomic number of 12. Atom I has 12 neutrons, atom II has 13 neutrons and atom III has 14 neutrons. Which of the following sentences is correct? A Atoms I, II and III are allotropes of each other. B Atoms I, II and III are isotopes of the same element. C Atom ...
... Three atoms, I, II and III, each have an atomic number of 12. Atom I has 12 neutrons, atom II has 13 neutrons and atom III has 14 neutrons. Which of the following sentences is correct? A Atoms I, II and III are allotropes of each other. B Atoms I, II and III are isotopes of the same element. C Atom ...
KEY Review Sheet: UNIT TWO TEST HISTORY OF ATOM
... Pauli Exclusion Principle is violated because in the 2p orbital, there are two electrons with the same spin. The two electrons should have opposite spins (one up arrow and one down arrow) Hund’s Rule is violated because in the 3p orbital there should not be two electrons. You should not give a singl ...
... Pauli Exclusion Principle is violated because in the 2p orbital, there are two electrons with the same spin. The two electrons should have opposite spins (one up arrow and one down arrow) Hund’s Rule is violated because in the 3p orbital there should not be two electrons. You should not give a singl ...
Chapter 4 - WordPress.com
... in which atoms lose energy by emitting radiation – Unstable elements do this until they are stable (often a different element) ...
... in which atoms lose energy by emitting radiation – Unstable elements do this until they are stable (often a different element) ...
Atomic Structure and Periodic Table Unit Notes Elements
... Elements- An element is either classified as a metal, nonmetal, or metalloid. The classification depends on the element’s location on the periodic table. Properties of Metals: o Metals are elements that have luster, conduct heat and electricity, and usually bend without breaking. Metals are also duc ...
... Elements- An element is either classified as a metal, nonmetal, or metalloid. The classification depends on the element’s location on the periodic table. Properties of Metals: o Metals are elements that have luster, conduct heat and electricity, and usually bend without breaking. Metals are also duc ...
PS 2.3
... this time. Let students know that they will be discussed in detail as each term is introduced. View ETV Streamline SC clip Energy and the Chemistry of Life – Atoms and Elements. This clip goes into the structure of the atom and the subatomic particles. Atoms and Elements 3:33 E1 5. Use handout one ( ...
... this time. Let students know that they will be discussed in detail as each term is introduced. View ETV Streamline SC clip Energy and the Chemistry of Life – Atoms and Elements. This clip goes into the structure of the atom and the subatomic particles. Atoms and Elements 3:33 E1 5. Use handout one ( ...
Basic Atomic Structure and Chemical Bonding Goals: Understand
... There are many elements that have multiple forms. Being the same element, they will all have the same number of protons. However, they have differing atomic masses, due to differences in their number of neutrons. Determining the number of subatomic particles of an Ion. Sometimes atoms will become ch ...
... There are many elements that have multiple forms. Being the same element, they will all have the same number of protons. However, they have differing atomic masses, due to differences in their number of neutrons. Determining the number of subatomic particles of an Ion. Sometimes atoms will become ch ...
1. Atoms and Bonding
... Other scans using radioactive tracers A hepatobiliary (HIDA) scan is an imaging procedure used to diagnose problems in the liver, gallbladder and bile ducts. http://www.youtube.com/watch?v=VDihrC 1RnYE ...
... Other scans using radioactive tracers A hepatobiliary (HIDA) scan is an imaging procedure used to diagnose problems in the liver, gallbladder and bile ducts. http://www.youtube.com/watch?v=VDihrC 1RnYE ...
document
... You need to keep track of how you are doing in the class and take action if you fall behind or have trouble with the material. A. Fellow students - meet others in the class. Even though you and the other student may be perplexed about a subject, you will find that talking together in the language of ...
... You need to keep track of how you are doing in the class and take action if you fall behind or have trouble with the material. A. Fellow students - meet others in the class. Even though you and the other student may be perplexed about a subject, you will find that talking together in the language of ...
Chapter 4 Notes
... In 1909, Rutherford carried out experiments that revealed an arrangement far different from Thomson’s model of the atom. The experimenters set up a lead-shielded box containing radioactive polonium, which emitted a beam of positively charged subatomic particles through a small hole. The sheet of gol ...
... In 1909, Rutherford carried out experiments that revealed an arrangement far different from Thomson’s model of the atom. The experimenters set up a lead-shielded box containing radioactive polonium, which emitted a beam of positively charged subatomic particles through a small hole. The sheet of gol ...
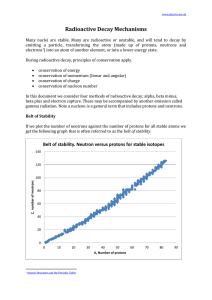
Radioactive Decay Mechanisms
... a helium nucleus; a group of two protons and two neutrons. A helium nucleus is very stable. An example of an alpha decay involves uranium-238: ...
... a helium nucleus; a group of two protons and two neutrons. A helium nucleus is very stable. An example of an alpha decay involves uranium-238: ...
I. Atoms are the smallest forms
... elements – Atoms changing identity • Chemical reactions do not effect the nucleus of an atom • Certain conditions can change the number of protons • Each atom has isotopes with different numbers of neutrons • Stability of nucleus depends on the right number of protons and neutrons • Too few or too m ...
... elements – Atoms changing identity • Chemical reactions do not effect the nucleus of an atom • Certain conditions can change the number of protons • Each atom has isotopes with different numbers of neutrons • Stability of nucleus depends on the right number of protons and neutrons • Too few or too m ...
Unit 2 * Chapter 11 - Dr. Wall`s Science
... • Sent positively charged particles through thin sheets of gold foil • Could see where the particles went after they went through the foil ...
... • Sent positively charged particles through thin sheets of gold foil • Could see where the particles went after they went through the foil ...
Name - cloudfront.net
... These are Dalton’s Postulate regarding the nature of the atom: 1. All matter consists of indivisible particles called atoms. 2. Atoms of the same element have the same shape and mass. 3. Atoms cannot be created or destroyed. 4. Atoms of different elements may combine with each other in fixed, simple ...
... These are Dalton’s Postulate regarding the nature of the atom: 1. All matter consists of indivisible particles called atoms. 2. Atoms of the same element have the same shape and mass. 3. Atoms cannot be created or destroyed. 4. Atoms of different elements may combine with each other in fixed, simple ...
Mass Defect (not in book)
... letters, can be used to construct many different words, the elements of the periodic table can be used to construct an almost infinite number of compounds. The earth is made of oxygen (49%), silicon (26%), aluminum (7.5 %), and a host of other elements. The human body is also made of a lot of oxygen ...
... letters, can be used to construct many different words, the elements of the periodic table can be used to construct an almost infinite number of compounds. The earth is made of oxygen (49%), silicon (26%), aluminum (7.5 %), and a host of other elements. The human body is also made of a lot of oxygen ...
Outline Chapter 10 The Periodic Law
... • The inert gases are inactive nonmetals. They are in group 8. 10-7. The Periodic Table Formulated by Russian chemist Dmitri Mendeleev in ~1869 Periodic law=states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular interv ...
... • The inert gases are inactive nonmetals. They are in group 8. 10-7. The Periodic Table Formulated by Russian chemist Dmitri Mendeleev in ~1869 Periodic law=states that when elements are listed in order of atomic number, elements with similar chemical and physical properties appear at regular interv ...
PowerPoint_Atomic Structure
... suggested that all matter was made up of tiny spheres that were able to bounce around with perfect elasticity and called them ...
... suggested that all matter was made up of tiny spheres that were able to bounce around with perfect elasticity and called them ...
Chapter 4 Atoms - Tangipahoa Parish School System
... • Isotope has same atomic number but different number of neutrons. • Isotopes have same atomic number but different mass numbers. • Isotopes have the same number of protons but different number of neutrons ...
... • Isotope has same atomic number but different number of neutrons. • Isotopes have same atomic number but different mass numbers. • Isotopes have the same number of protons but different number of neutrons ...