problems - chem.msu.su
... 4. The potential corresponding to the reduction of complex MetLx is described with equations: E = E½(Metn+) + 0.059/n·lg[Metn+] = E½(MetLx) + 0.059/n·lg[MetLx], where E½(Metn+) and E½(MetLx) are the half-wave potentials for the free and complexed metalions, respectively; [MetLx] and [Metn+] are the ...
... 4. The potential corresponding to the reduction of complex MetLx is described with equations: E = E½(Metn+) + 0.059/n·lg[Metn+] = E½(MetLx) + 0.059/n·lg[MetLx], where E½(Metn+) and E½(MetLx) are the half-wave potentials for the free and complexed metalions, respectively; [MetLx] and [Metn+] are the ...
File
... Identify one isomer that will react with aqueous sodium hydroxide almost exclusively by an SN2 mechanism. Draw the mechanism for this reaction using curly arrows to represent the movement of electron pairs. Include the structural formulas of the transition state and the organic product. ...
... Identify one isomer that will react with aqueous sodium hydroxide almost exclusively by an SN2 mechanism. Draw the mechanism for this reaction using curly arrows to represent the movement of electron pairs. Include the structural formulas of the transition state and the organic product. ...
A.P. Chemistry Writing Chemical Reactions Generally students do
... present. The most significant hurdle with these reactions is likely to be the organic compound names. If you don’t know what propanal is then it doesn’t matter that it is easy to write the combustion reaction. While the compounds selected are seldom complicated, some knowledge of basic organic funct ...
... present. The most significant hurdle with these reactions is likely to be the organic compound names. If you don’t know what propanal is then it doesn’t matter that it is easy to write the combustion reaction. While the compounds selected are seldom complicated, some knowledge of basic organic funct ...
Topic 1 Review - Capital High School
... period of the periodic table, and __________ as you go from the bottom to the top of a group in the table. 35. In general, as you go across a period in the periodic table from left to right: (1) the atomic radius __________; (2) the electron affinity becomes __________ negative; and (3) the first io ...
... period of the periodic table, and __________ as you go from the bottom to the top of a group in the table. 35. In general, as you go across a period in the periodic table from left to right: (1) the atomic radius __________; (2) the electron affinity becomes __________ negative; and (3) the first io ...
Writing Chemical Reactions
... present. The most significant hurdle with these reactions is likely to be the organic compound names. If you don’t know what propanal is then it doesn’t matter that it is easy to write the combustion reaction. While the compounds selected are seldom complicated, some knowledge of basic organic funct ...
... present. The most significant hurdle with these reactions is likely to be the organic compound names. If you don’t know what propanal is then it doesn’t matter that it is easy to write the combustion reaction. While the compounds selected are seldom complicated, some knowledge of basic organic funct ...
5H2O → CuSO4 + 5H2O(g)
... from aqueous solutions ◦ Acid-base neutralization: acid and base react to form water and a salt (ionic compound) ◦ Oxidation-Reduction: electrons are transferred between atoms in reaction Combination Decomposition Single-replacement (metal or hydrogen) ...
... from aqueous solutions ◦ Acid-base neutralization: acid and base react to form water and a salt (ionic compound) ◦ Oxidation-Reduction: electrons are transferred between atoms in reaction Combination Decomposition Single-replacement (metal or hydrogen) ...
Problem Set: Empirical and Molecular Formulas
... had 152.5 g CO and 24.50 g H2, how many kilograms of CH3OH would be produced? (Hint: make sure equation is balanced first!) ...
... had 152.5 g CO and 24.50 g H2, how many kilograms of CH3OH would be produced? (Hint: make sure equation is balanced first!) ...
Lectures 5
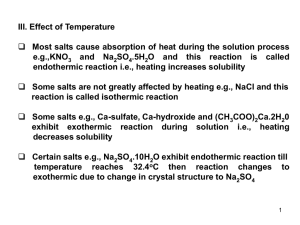
... Some salts e.g., Ca-sulfate, Ca-hydroxide and (CH3COO)2Ca.2H20 exhibit exothermic reaction during solution i.e., heating decreases solubility Certain salts e.g., Na2SO4.10H2O exhibit endothermic reaction till temperature reaches 32.4oC then reaction changes to exothermic due to change in crystal ...
... Some salts e.g., Ca-sulfate, Ca-hydroxide and (CH3COO)2Ca.2H20 exhibit exothermic reaction during solution i.e., heating decreases solubility Certain salts e.g., Na2SO4.10H2O exhibit endothermic reaction till temperature reaches 32.4oC then reaction changes to exothermic due to change in crystal ...
Introduction
... Which one is more likely to be pulled apart by water molecules? Electrolytes are ionic and strong acid solutions (e.g., GatoradeTM); Nonelectrolytes are covalent compounds (e.g., sugar); weak electrolytes are in between. ...
... Which one is more likely to be pulled apart by water molecules? Electrolytes are ionic and strong acid solutions (e.g., GatoradeTM); Nonelectrolytes are covalent compounds (e.g., sugar); weak electrolytes are in between. ...
model paper-1 - WordPress.com
... Name four quantum numbers and what are their significance. 5M OR a) State Heisenberg Uncertainty principle. b) What is the energy in joules, required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit, and what is the wavelength of the light emitted when the ...
... Name four quantum numbers and what are their significance. 5M OR a) State Heisenberg Uncertainty principle. b) What is the energy in joules, required to shift the electron of the hydrogen atom from the first Bohr orbit to the fifth Bohr orbit, and what is the wavelength of the light emitted when the ...
Click to download. - Life Learning Cloud
... There are 4 main structures which substances can have. These are known as: SIMPLE MOLECULAR., GIANT COVALENT, GIANT IONIC and GIANT METALLIC Simple Molecular. Simple molecular substances have small molecules, such as H2O or CO2. The atoms in these molecules are held together by strong forces called ...
... There are 4 main structures which substances can have. These are known as: SIMPLE MOLECULAR., GIANT COVALENT, GIANT IONIC and GIANT METALLIC Simple Molecular. Simple molecular substances have small molecules, such as H2O or CO2. The atoms in these molecules are held together by strong forces called ...
The Copper Cycle
... Acids and Bases In general, acids are compounds that produce hydrogen ions (H+), also called protons. Acids can also be defined as substances that produce hydronium ions (H3O+), which is a hydrogen ion combined with a water molecule: H+(aq) + H2O(l) → H3O+(aq). The two equations below both represent ...
... Acids and Bases In general, acids are compounds that produce hydrogen ions (H+), also called protons. Acids can also be defined as substances that produce hydronium ions (H3O+), which is a hydrogen ion combined with a water molecule: H+(aq) + H2O(l) → H3O+(aq). The two equations below both represent ...
AP CHEMISTRY MRS. SPENCER CHAPTER 4 TEST: SOLUTION
... Answer Question 4 below. The Section II score weighting for this question is 10 percent. (Note: I have included below only 1 of the 3 reactions listed in this question.) 4. For (each of) the following (three) reaction(s), in part (i) write a balanced equation for the reaction and in part (ii) answer ...
... Answer Question 4 below. The Section II score weighting for this question is 10 percent. (Note: I have included below only 1 of the 3 reactions listed in this question.) 4. For (each of) the following (three) reaction(s), in part (i) write a balanced equation for the reaction and in part (ii) answer ...
Synthesis Reactions occur when two of more reactants combine to
... 5. Hydrogen peroxide decomposes into oxygen and water. example 2H2O2 2H2O + O2 6. Ammonium carbonate decomposes into ammonia, carbon dioxide, and water. example A sample of ammonium carbonate is heated. (NH4)2CO3 2NH3 + CO2 + H2O 7. Sulfurous acid decomposes into sulfur dioxide and water. exam ...
... 5. Hydrogen peroxide decomposes into oxygen and water. example 2H2O2 2H2O + O2 6. Ammonium carbonate decomposes into ammonia, carbon dioxide, and water. example A sample of ammonium carbonate is heated. (NH4)2CO3 2NH3 + CO2 + H2O 7. Sulfurous acid decomposes into sulfur dioxide and water. exam ...
Synthesis of Alum Lab
... d) Alum forms from potassium ions, hydrated aluminum ions sulfate ions and water: K+(aq) + 6H2O(l) + 2SO42-(aq) + [Al(H2O)6]3+(aq) KAl(SO4)2.12H2O(s) ...
... d) Alum forms from potassium ions, hydrated aluminum ions sulfate ions and water: K+(aq) + 6H2O(l) + 2SO42-(aq) + [Al(H2O)6]3+(aq) KAl(SO4)2.12H2O(s) ...
Review Final 111 Lect
... (Hint: You need to write the equilibrium equation for the solubility of CaF2 given above) 37. When barium chloride is added to a saturated solution of BaSO4(s), which of the following will result?(Hint: Write the equilibrium equation for the solubility of BaSO4 (s).) a. The concentration of SO42- wi ...
... (Hint: You need to write the equilibrium equation for the solubility of CaF2 given above) 37. When barium chloride is added to a saturated solution of BaSO4(s), which of the following will result?(Hint: Write the equilibrium equation for the solubility of BaSO4 (s).) a. The concentration of SO42- wi ...
spring semester review
... d) The reaction is not spontaneous at any temperatures e) We cannot tell from the information given 59. What is the reducing agent in following reaction: Cr2O72- + 6S2O32- + 14H+ --> 2Cr3+ + 3S4O62- + 7H20 a) Cr2O72b) S2O32c) H+ d) Cr3+ e) S4O6260. Which substance is the oxidizing agent in the follo ...
... d) The reaction is not spontaneous at any temperatures e) We cannot tell from the information given 59. What is the reducing agent in following reaction: Cr2O72- + 6S2O32- + 14H+ --> 2Cr3+ + 3S4O62- + 7H20 a) Cr2O72b) S2O32c) H+ d) Cr3+ e) S4O6260. Which substance is the oxidizing agent in the follo ...