equilibrium
... 2. What is the significance of double arrows in an equation? 3. How is the term equilibrium used to describe an aqueous solution which is saturated? 4. How do the rates of forward and reverse reactions compare at a state of dynamic equilibrium? 5. How do the concentrations (or amounts) of reactants ...
... 2. What is the significance of double arrows in an equation? 3. How is the term equilibrium used to describe an aqueous solution which is saturated? 4. How do the rates of forward and reverse reactions compare at a state of dynamic equilibrium? 5. How do the concentrations (or amounts) of reactants ...
Worksheet Key
... a. The reaction is finished, no more products are forming. __ false __ b. The concentrations of the reactants and the products are equal. false __ c. The concentrations are no longer changing. _ true _ d. The reaction is not over, but will continue forever if isolated. _ true _ e. The speed at which ...
... a. The reaction is finished, no more products are forming. __ false __ b. The concentrations of the reactants and the products are equal. false __ c. The concentrations are no longer changing. _ true _ d. The reaction is not over, but will continue forever if isolated. _ true _ e. The speed at which ...
towards the synthesis of functionalised macrocyclic receptors
... Jean-Marie Lehn described supramolecular chemistry as an interdisciplinary field of science covering the chemical, physical and biological features of highly complex chemical species involving two or more molecules held together by noncovalent interactions.1 Therefore, two factors which are importan ...
... Jean-Marie Lehn described supramolecular chemistry as an interdisciplinary field of science covering the chemical, physical and biological features of highly complex chemical species involving two or more molecules held together by noncovalent interactions.1 Therefore, two factors which are importan ...
2015 chemistry
... Explain why the percentage of oil converted in an enzyme-catalysed reaction is very low at high temperatures. _______________________________________________________________________________________________________ ______________________________________________________________________________________ ...
... Explain why the percentage of oil converted in an enzyme-catalysed reaction is very low at high temperatures. _______________________________________________________________________________________________________ ______________________________________________________________________________________ ...
chapter 21 chemistry of the main-group elements i
... bond them together. To bond these four atoms into a chain requires three electron pairs. Since each electron pair in a bridging bond replaces two “normal” bonds, there must be at least two bridging bonds in the B4 H10 molecules. By analogy with B2 H 6 , we might write the structure below left. But t ...
... bond them together. To bond these four atoms into a chain requires three electron pairs. Since each electron pair in a bridging bond replaces two “normal” bonds, there must be at least two bridging bonds in the B4 H10 molecules. By analogy with B2 H 6 , we might write the structure below left. But t ...
chemistry writing team
... SOME BASIC CONCEPTS OF CHEMISTRY Law of conservation of mass : ‘Mass can neither be created nor destroyed.’ In all physical and chemical changes, the total mass of reactants is equal to that of products. Law of constant composition : A chemical compound is always found to be made of same elements co ...
... SOME BASIC CONCEPTS OF CHEMISTRY Law of conservation of mass : ‘Mass can neither be created nor destroyed.’ In all physical and chemical changes, the total mass of reactants is equal to that of products. Law of constant composition : A chemical compound is always found to be made of same elements co ...
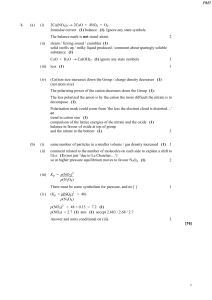
1. (a) (i) 2Ca(NO3)2 → 2CaO + 4NO2 + O2 formulae correct (1
... This could be shown on the graph because pH = pKa when [CH3COO−] = [CH3COOH] (1) ...
... This could be shown on the graph because pH = pKa when [CH3COO−] = [CH3COOH] (1) ...
Slides
... Japanese media and officials have reported. Kyodo News Service and public broadcaster NHK reported that four workers had died after suffering severe burns, and that at least one other worker was in critical condition. Those who died were exposed to steam as hot as 200 Celsius (392 Fahrenheit), offic ...
... Japanese media and officials have reported. Kyodo News Service and public broadcaster NHK reported that four workers had died after suffering severe burns, and that at least one other worker was in critical condition. Those who died were exposed to steam as hot as 200 Celsius (392 Fahrenheit), offic ...
AP Exam Review Questions
... is increased, which way would the system shift to relieve the stress? N2 (g) + 3H2 (g) ↔ 2NH3 (g) + heat Ans: The reaction would shift to the left to reduce the heat. ...
... is increased, which way would the system shift to relieve the stress? N2 (g) + 3H2 (g) ↔ 2NH3 (g) + heat Ans: The reaction would shift to the left to reduce the heat. ...
KEY + + - UIC Department of Chemistry
... of product which you believe to be N2 . Is that possible? Explain your answer. (7 points) mass N2 possible = (0.0880778 mol NH3)(2 mol N2/4 mol NH3)(28.0134 g/1 mol N2) = 1.23 g N 2 (theoretical yield) Not possible to form 1.80 g N2. Can't make more N2 than the theoretical yield. ...
... of product which you believe to be N2 . Is that possible? Explain your answer. (7 points) mass N2 possible = (0.0880778 mol NH3)(2 mol N2/4 mol NH3)(28.0134 g/1 mol N2) = 1.23 g N 2 (theoretical yield) Not possible to form 1.80 g N2. Can't make more N2 than the theoretical yield. ...
Version A
... 5. If the temperature increases from 10°C to 60°C at a constant pressure of 0.4 atmosphere, which of the processes occurs? ...
... 5. If the temperature increases from 10°C to 60°C at a constant pressure of 0.4 atmosphere, which of the processes occurs? ...
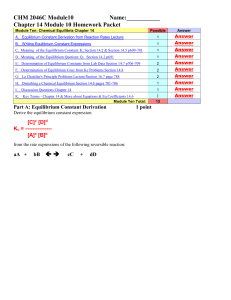
CHEM 1212 Module Ten-Chapter 16 Name
... 4. Your textbook introduces the concept of equilibrium by noting that no reaction goes fully to completion. What does this imply about the reverse reaction? ...
... 4. Your textbook introduces the concept of equilibrium by noting that no reaction goes fully to completion. What does this imply about the reverse reaction? ...
1.2 Calculations
... Indicators are generally weak acids so we only add a few drops of them. If too much is added it will affect the titration result Distilled water can be added to the conical flask during a titration to wash the sides of the flask so that all the acid on the side is washed into the reaction mixture to ...
... Indicators are generally weak acids so we only add a few drops of them. If too much is added it will affect the titration result Distilled water can be added to the conical flask during a titration to wash the sides of the flask so that all the acid on the side is washed into the reaction mixture to ...