
Chemical Equilibrium - The Gurukul Institute
... 1. Ammonium hydrogen sulphite dissociates as follows NH4HS(s) ⇌NH3(g) + H2S(g) If solid NH4HS is placed in an evacuated flask at certain temperature it will dissociate until the total pressure is 600 torr. (A) calculate the value of equilibrium constant for the dissociation reaction (B) Additional N ...
... 1. Ammonium hydrogen sulphite dissociates as follows NH4HS(s) ⇌NH3(g) + H2S(g) If solid NH4HS is placed in an evacuated flask at certain temperature it will dissociate until the total pressure is 600 torr. (A) calculate the value of equilibrium constant for the dissociation reaction (B) Additional N ...
Chemistry - Set as Home Page
... Oxygen from the air is used by all living things in the process called _________. Oxygen returned to the air by plants in a process called _________. ...
... Oxygen from the air is used by all living things in the process called _________. Oxygen returned to the air by plants in a process called _________. ...
Acids, bases and combustion
... (a) Covalent bond is bond between non-metal atoms where shared electrons are donated equally by all the atoms involved. Dative bond is a bond in which shared electrons are donated by one atom. ½ bond in nitrogen requires very high temperatures to break (b) The presence of triple (a) Reduction by u ...
... (a) Covalent bond is bond between non-metal atoms where shared electrons are donated equally by all the atoms involved. Dative bond is a bond in which shared electrons are donated by one atom. ½ bond in nitrogen requires very high temperatures to break (b) The presence of triple (a) Reduction by u ...
Acknowledgements - HAL
... mg.L-1, the time to reach equilibrium was shown to be rapid, i.e. below five minutes. For PAA at concentrations <1 mg.L-1, contact times varying from 1 and 7 days were necessary for equilibration. Eu, Cm and PAA, Ac concentrations were checked using a PQ-Excel ICP-MS (VG-Elemental), by liquid scinti ...
... mg.L-1, the time to reach equilibrium was shown to be rapid, i.e. below five minutes. For PAA at concentrations <1 mg.L-1, contact times varying from 1 and 7 days were necessary for equilibration. Eu, Cm and PAA, Ac concentrations were checked using a PQ-Excel ICP-MS (VG-Elemental), by liquid scinti ...
Collected Essays chapter 13 answers
... If the temperature is increased for an endothermic reaction ( ΔH◦298 = +131 kJ mol−1), then by Le Chatelier’s principle the reaction will shift toward products, thereby absorbing energy. With greater concentrations of products at equilibrium, the value of Kp will increase. (d) For reaction Y at 298 ...
... If the temperature is increased for an endothermic reaction ( ΔH◦298 = +131 kJ mol−1), then by Le Chatelier’s principle the reaction will shift toward products, thereby absorbing energy. With greater concentrations of products at equilibrium, the value of Kp will increase. (d) For reaction Y at 298 ...
EDTA Titrations
... buffer the pH to (a) 10.0. What is aY4- ? (b) What is aY4- if the pH of the solution is buffered to 11.0? ...
... buffer the pH to (a) 10.0. What is aY4- ? (b) What is aY4- if the pH of the solution is buffered to 11.0? ...
IChO_Comp_Prob_Answ 1997
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
... routine material studied in most high schools around the world. But this is how it should be since the competitors involved are among the best that our countries have to offer. However, it is felt that even these topics and the level of expertise expected can be mastered by our students without sign ...
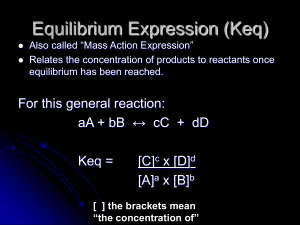
Equilibrium Expression (Keq)
... http://www.kentchemistry.com/links/Kinetics/EquilibriumExpression.htm ...
... http://www.kentchemistry.com/links/Kinetics/EquilibriumExpression.htm ...
(K c ) [A] - Knockhardy
... the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board is available. Accompanying notes on this, and the full range of AS and A2 topics, ...
... the AQA and OCR specifications but is suitable for other examination boards. Individual students may use the material at home for revision purposes or it may be used for classroom teaching if an interactive white board is available. Accompanying notes on this, and the full range of AS and A2 topics, ...
5 organic chemistry: functional groups
... If the crystallizing dish in the upper right corner is moved into the center of the projector, however, the color of the bromine slowly disappears. This can be explained by noting that alkanes react with halogens at high temperatures or in the presence of light to form alkyl halides, as noted in Sec ...
... If the crystallizing dish in the upper right corner is moved into the center of the projector, however, the color of the bromine slowly disappears. This can be explained by noting that alkanes react with halogens at high temperatures or in the presence of light to form alkyl halides, as noted in Sec ...
answer ch6 - Mr Khaled Nasr
... (9) The weight of a substance in 100 grams of its solution. (10)The number of moles of solute per liter of solution. (11)A solution containing 1 mole of solute in 1000mL of solution. (12)A method of quantitative analysis that is based on measurement of the volume of the substance to be analyzed. (13 ...
... (9) The weight of a substance in 100 grams of its solution. (10)The number of moles of solute per liter of solution. (11)A solution containing 1 mole of solute in 1000mL of solution. (12)A method of quantitative analysis that is based on measurement of the volume of the substance to be analyzed. (13 ...
Chemistry of CHLORINE
... Electronegativity is the ease/tendency of gaining/ acquiring electrons by an element during chemical reactions. It does not involve use of energy but theoretical arbitrary Pauling’s scale of measurements. (g) (i) 5cm3 of sodium chloride, Sodium bromide and Sodium iodide solutions were put separately ...
... Electronegativity is the ease/tendency of gaining/ acquiring electrons by an element during chemical reactions. It does not involve use of energy but theoretical arbitrary Pauling’s scale of measurements. (g) (i) 5cm3 of sodium chloride, Sodium bromide and Sodium iodide solutions were put separately ...
TOPIC 11 Further equilibrium 11.1 Chemical equilibrium
... Make sure the equivalence point is at a pH greater than 7, since this is a titration of a weak acid and a strong base. Make sure the finishing pH is realistic for the concentration of base taken. The curve would start at a higher pH. The volume of sodium hydroxide at the equivalence point would be 1 ...
... Make sure the equivalence point is at a pH greater than 7, since this is a titration of a weak acid and a strong base. Make sure the finishing pH is realistic for the concentration of base taken. The curve would start at a higher pH. The volume of sodium hydroxide at the equivalence point would be 1 ...
Experimental Chemistry I
... During the course of the dissolving process, MnO2 as a by-product is formed, which must be removed; • warm a funnel and filter by pouring preheated distilled water through the glass-wool popped funnel; • start the actual filtration procedure with the heated KMnO2 / KMnO2-solution; Note: to avoid any ...
... During the course of the dissolving process, MnO2 as a by-product is formed, which must be removed; • warm a funnel and filter by pouring preheated distilled water through the glass-wool popped funnel; • start the actual filtration procedure with the heated KMnO2 / KMnO2-solution; Note: to avoid any ...
Part-1
... Osmotic pressure of a solution is directly proportional to the number of moles of solute dissolved per litre of solution at a given temperature. Solutions having equal molar concentration and equal osmotic pressure at a given temperature are called isotonic solutions, e.g., A 0.90% (mass/volume) sol ...
... Osmotic pressure of a solution is directly proportional to the number of moles of solute dissolved per litre of solution at a given temperature. Solutions having equal molar concentration and equal osmotic pressure at a given temperature are called isotonic solutions, e.g., A 0.90% (mass/volume) sol ...
Chemical Equilibrium - Shailendra Kumar Chemistry
... 2C + D was studied using an initial concentration of B which was 1.5 more that of A. But the equilibrium concentration of A and C were found to be equal. Then the Kc for the equilibrium is : (a) 4 ...
... 2C + D was studied using an initial concentration of B which was 1.5 more that of A. But the equilibrium concentration of A and C were found to be equal. Then the Kc for the equilibrium is : (a) 4 ...
General and Inorganic Chemistry – Laboratory Techniques
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...
... Knowledge of students on Chemistry at the beginning of their graduate studies is rather different. Most of the students do not have proper laboratory expertise. This educational experience prompted the faculty of the institute to compile an educational material that can help students to make themsel ...








![(K c ) [A] - Knockhardy](http://s1.studyres.com/store/data/011755527_1-914ea907d1ff7656ef398ad87316c94c-300x300.png)













