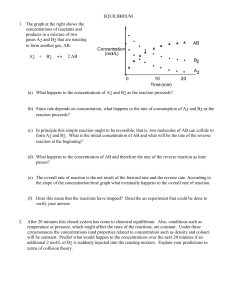
CHEMICAL EQUILIBRIUM
... PCl5 (g). after the system had reached equilibrium, 2.00 x 10 -3 mol Cl2 (g) was found in the flask. Gaseous PCl5 decomposes according to the following reaction: PCl5 (g) ↔ PCl3 (g) + Cl2 (g) Calculate the equilibrium concentrations of all species and the value of K. The expression for K is: ...
... PCl5 (g). after the system had reached equilibrium, 2.00 x 10 -3 mol Cl2 (g) was found in the flask. Gaseous PCl5 decomposes according to the following reaction: PCl5 (g) ↔ PCl3 (g) + Cl2 (g) Calculate the equilibrium concentrations of all species and the value of K. The expression for K is: ...
QualGroupB - Back To Home Page
... boiling water bath. If any solid remains, add a few more drops of the HCl. Centrifuge and, if any precipitate remains, decant the supernatant into a test tube LABELED B1 TEST SOLUTION. Discard any remaining precipitate. ...
... boiling water bath. If any solid remains, add a few more drops of the HCl. Centrifuge and, if any precipitate remains, decant the supernatant into a test tube LABELED B1 TEST SOLUTION. Discard any remaining precipitate. ...
Exam Review Packet Table of Contents
... An incorrect statement in an otherwise correct 2 pt response will result in a score of 1 pt The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The ...
... An incorrect statement in an otherwise correct 2 pt response will result in a score of 1 pt The answers labeled (i) below received two points; (ii) received one point. a) two points -‐ The ...
Equilibrium - chemmybear.com
... (d) The mass of NH4HS decreases because the endothermic reaction absorbs heat and goes nearer to completion (to the right) as the temperature increases. 1992 A 2 NaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g) Solid sodium hydrogen carbonate, NaHCO3, decomposes on heating according to the equation above. (a ...
... (d) The mass of NH4HS decreases because the endothermic reaction absorbs heat and goes nearer to completion (to the right) as the temperature increases. 1992 A 2 NaHCO3(s) Na2CO3(s) + H2O(g) + CO2(g) Solid sodium hydrogen carbonate, NaHCO3, decomposes on heating according to the equation above. (a ...
chemical and isotopic evidence for the in situ origin of marine humic
... into the humic acid by the NaO,I-I treatment. However, the samples from Saanich Inlet and the Dead Sea had all elemental sulfur extracted by solvents and the unstable sulfides removed by acid treatment bcforc the humic acid extraction, and the humic acid was still rich in sulfur. The samples rich in ...
... into the humic acid by the NaO,I-I treatment. However, the samples from Saanich Inlet and the Dead Sea had all elemental sulfur extracted by solvents and the unstable sulfides removed by acid treatment bcforc the humic acid extraction, and the humic acid was still rich in sulfur. The samples rich in ...
Relating Solubility and Ksp
... By looking at this expression we can see that the molar expression is 1 and considering that the concentrations of these ions are small, we are able to say that the activity of the ions are equal to their molar concentrations which equals 1. Now we can see that Ksp is equal to the product of the ion ...
... By looking at this expression we can see that the molar expression is 1 and considering that the concentrations of these ions are small, we are able to say that the activity of the ions are equal to their molar concentrations which equals 1. Now we can see that Ksp is equal to the product of the ion ...
Atomic Structure
... The statements (i) “In filling a group of orbitals of equal energy it is energetically preferable to assign electrons to empty orbitals rather than pair them into a particular orbital. (ii) When two electrons are placed in two different orbitals, energy is lower if the espins are parallel” are valid ...
... The statements (i) “In filling a group of orbitals of equal energy it is energetically preferable to assign electrons to empty orbitals rather than pair them into a particular orbital. (ii) When two electrons are placed in two different orbitals, energy is lower if the espins are parallel” are valid ...
4.2- Reaction Stoichiometry Reaction Stoichiometry
... limes, and vinegar. Vitamin C and aspirin are also acids ...
... limes, and vinegar. Vitamin C and aspirin are also acids ...
5 SURFACE CHEMISTRY CATEGORY
... 3.Define the term osmotic pressure. Describe how the molecular mass of a substance can be determined by a method based on measurement of osmotic pressure? 4.Define osmotic pressure. How is it that measurement of osmotic pressures is more widely used for determining molar masses of macromolecules tha ...
... 3.Define the term osmotic pressure. Describe how the molecular mass of a substance can be determined by a method based on measurement of osmotic pressure? 4.Define osmotic pressure. How is it that measurement of osmotic pressures is more widely used for determining molar masses of macromolecules tha ...
Unusually Strong Dependence of Conformation on Solvent
... has a greater tendency to extract from aqueous methanol into pentane. This represents a greater hydrophobicity of the cis, whereas the trans stereoisomer is more hydrophilic. The partition coefficients can differ by more than a factor of 10, quite a large value for molecules that are merely stereois ...
... has a greater tendency to extract from aqueous methanol into pentane. This represents a greater hydrophobicity of the cis, whereas the trans stereoisomer is more hydrophilic. The partition coefficients can differ by more than a factor of 10, quite a large value for molecules that are merely stereois ...
Abstract - Engineering | UMass
... the protein backbone) occurs only after many days and in the presence of a long-lasting chlorine residual. Based on the protein-chlorine reactions, DHAN should be characterized by slow chlorine-dependent formation, which occurs at higher rates as the pH increases. Actual observations with natural wa ...
... the protein backbone) occurs only after many days and in the presence of a long-lasting chlorine residual. Based on the protein-chlorine reactions, DHAN should be characterized by slow chlorine-dependent formation, which occurs at higher rates as the pH increases. Actual observations with natural wa ...
ExamView - 1984 AP Chemistry Exam.tst
... (1) Test Questions are Copyright © 1984-2002 by College Entrance Examination Board, Princeton, NJ. All rights reserved. For face-to-face teaching purposes, classroom teachers are permitted to reproduce the questions. Web or Mass distribution prohibited. (2) AP® is a registered trademark of the Colle ...
... (1) Test Questions are Copyright © 1984-2002 by College Entrance Examination Board, Princeton, NJ. All rights reserved. For face-to-face teaching purposes, classroom teachers are permitted to reproduce the questions. Web or Mass distribution prohibited. (2) AP® is a registered trademark of the Colle ...
Chemistry JAMB Past Questions
... H2SO4 B. The solution was concentrated C. When the concentrate was cooled, crystals formed were removed by filtration. D. The crystals were washed with very cold water E. The crystals were then allowed to dry. ...
... H2SO4 B. The solution was concentrated C. When the concentrate was cooled, crystals formed were removed by filtration. D. The crystals were washed with very cold water E. The crystals were then allowed to dry. ...
Worksheet # 1 Solubility and Saturated Solutions 1. Define and give
... When excess Ag2CO3(s) is shaken with 1.00 L of 0.200 M K2CO3 it is determined that 6.00 x 10-6 moles of Ag2CO3 dissolves. Calculate the solubility product of Ag2CO3. ...
... When excess Ag2CO3(s) is shaken with 1.00 L of 0.200 M K2CO3 it is determined that 6.00 x 10-6 moles of Ag2CO3 dissolves. Calculate the solubility product of Ag2CO3. ...
WRITING CHEMICAL FORMULAE
... measure out a volume of the solution, we want to know how much acid it contains. To do this, we need to define the CONCENTRATION of the solution. This tells us how much solute there is in a fixed volume of the solution. In chemistry, “how much” means MOLES and the “fixed volume” is taken as 1 litre ...
... measure out a volume of the solution, we want to know how much acid it contains. To do this, we need to define the CONCENTRATION of the solution. This tells us how much solute there is in a fixed volume of the solution. In chemistry, “how much” means MOLES and the “fixed volume” is taken as 1 litre ...
Amines(Chapter 13)
... Secondary amines react with Hinsberg's reagent to form a product that is insoluble in an alkali. For example, N, N - diethylamine reacts with Hinsberg's reagent to form N, N diethylbenzenesulphonamide, which is insoluble in an alkali. Tertiary amines, however, do not react with Hinsberg's reagent. ( ...
... Secondary amines react with Hinsberg's reagent to form a product that is insoluble in an alkali. For example, N, N - diethylamine reacts with Hinsberg's reagent to form N, N diethylbenzenesulphonamide, which is insoluble in an alkali. Tertiary amines, however, do not react with Hinsberg's reagent. ( ...
AP Chemistry Notes and Worksheets 2014
... covalent bonds-created when two or more nonmetals share electrons molecule- atoms held together by covalent bonds ions- charged particles formed by the loss or gain of electrons ionic bonds- compounds created when one atom loses an electron and another gains it; are held together by electros ...
... covalent bonds-created when two or more nonmetals share electrons molecule- atoms held together by covalent bonds ions- charged particles formed by the loss or gain of electrons ionic bonds- compounds created when one atom loses an electron and another gains it; are held together by electros ...