Volatility of Organic Aerosol: Evaporation of Ammonium Sulfate
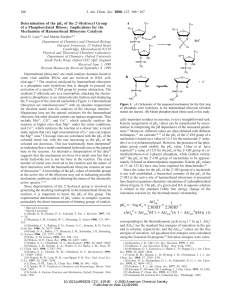
... over a mixed particle surface are affected by the particle composition. This effect is described by the activity, i.e. the product of the activity coefficient and the molar fraction of the given compound in the particle. For aqueous solutions of single organic compounds directly measurement-based activi ...
... over a mixed particle surface are affected by the particle composition. This effect is described by the activity, i.e. the product of the activity coefficient and the molar fraction of the given compound in the particle. For aqueous solutions of single organic compounds directly measurement-based activi ...
Packet 1 - Kentucky Community and Technical College System
... Because water is so abundant it is very useful to use as a solvent. The fact that so many ionic and molecular chemicals are soluble in it makes it even more useful. ...
... Because water is so abundant it is very useful to use as a solvent. The fact that so many ionic and molecular chemicals are soluble in it makes it even more useful. ...
Hydroxyl Group of a Phosphorylated Ribose
... metal ions in the reaction. It has been established, based on atomic substitution of the pro-R and pro-S oxygens by sulfurs (the thioeffect30) and by spectroscopic studies,31 that a metal ion coordinates directly with the pro-R oxygen of the phosphate at the active site.31 Kinetic studies of hammerh ...
... metal ions in the reaction. It has been established, based on atomic substitution of the pro-R and pro-S oxygens by sulfurs (the thioeffect30) and by spectroscopic studies,31 that a metal ion coordinates directly with the pro-R oxygen of the phosphate at the active site.31 Kinetic studies of hammerh ...
Solubility
... Observation: If NaNO3 salt is added to AgCl precipitate, it’s solubility can be increased dramatically. There is no chemical reaction with the NaNO3, so what is going on? Thus far we have used molar concentrations in Ksp and other equilibrium expressions, but this is an approximation of the exact so ...
... Observation: If NaNO3 salt is added to AgCl precipitate, it’s solubility can be increased dramatically. There is no chemical reaction with the NaNO3, so what is going on? Thus far we have used molar concentrations in Ksp and other equilibrium expressions, but this is an approximation of the exact so ...
Exam 1
... Both salicylic acid and aspirin can be detected using ultraviolet light. Ethyl ethanoate can be used as a suitable solvent. ...
... Both salicylic acid and aspirin can be detected using ultraviolet light. Ethyl ethanoate can be used as a suitable solvent. ...
The Study of pH Influence on Bovine Liver Catalase by Means of UV
... both toward acidic and alkaline environment causes decrease of correlation time value (Wc = 1.21*10-10 for pH 2, Wc = 1.84*10-10 for pH 4, Wc = 3.41*10-10 for pH 6, Wc = 3.11*10-10 for pH 8, Wc = 2.13*10-10 for pH 10). The decrease of the correlation time to about 40% of the Wc obtained for native c ...
... both toward acidic and alkaline environment causes decrease of correlation time value (Wc = 1.21*10-10 for pH 2, Wc = 1.84*10-10 for pH 4, Wc = 3.41*10-10 for pH 6, Wc = 3.11*10-10 for pH 8, Wc = 2.13*10-10 for pH 10). The decrease of the correlation time to about 40% of the Wc obtained for native c ...
CHEM181H1_06_2013_Y_P1
... or instrument, the use of which is not authorized by the examiner or the examination officer. (b) they possess, use, or attempt to use during an examination, any paper, book, note, document or instrument the use of which is not authorized by the examiner or the examination officer. (c) they remov ...
... or instrument, the use of which is not authorized by the examiner or the examination officer. (b) they possess, use, or attempt to use during an examination, any paper, book, note, document or instrument the use of which is not authorized by the examiner or the examination officer. (c) they remov ...
SCH 4U REVIEW Notes
... catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical characteristics to that molecule – there are three main components: multiple bo ...
... catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical characteristics to that molecule – there are three main components: multiple bo ...
Boronic acids facilitate rapid oxime condensations at neutral pH
... materials. There are commercial libraries of phenylboronic acid and boronic ester compounds, many of which contain an aldehyde or can be trivially elaborated to incorporate one. Furthermore, the widespread use of oxime conjugations for connective processes at high concentration means that a variety ...
... materials. There are commercial libraries of phenylboronic acid and boronic ester compounds, many of which contain an aldehyde or can be trivially elaborated to incorporate one. Furthermore, the widespread use of oxime conjugations for connective processes at high concentration means that a variety ...
Liquid-phase hydrodechlorination of chlorobenzene by molecular
... such as reagent solubility, substrate activation due to coordination interactions, and processes of adsorption–desorption of the reagents and products onto a catalyst surface. When comparing the results of research on liquid-phase catalytic hydrodehalogenation (HDG) of different halogenated aromatic ...
... such as reagent solubility, substrate activation due to coordination interactions, and processes of adsorption–desorption of the reagents and products onto a catalyst surface. When comparing the results of research on liquid-phase catalytic hydrodehalogenation (HDG) of different halogenated aromatic ...
Corrosion Inhibition of Carbon Steel in 1M HCl Solution by
... Two types of Schiff bases were prepared throughout condensation of 1,3-diaminopropane with two different types from Salicyaldehyde and furfuraldehyde. The chemical structures of the prepared Schiff bases were confirmed by using elemental analysis and FT-IR spectra. The synthesized Schiff bases were ...
... Two types of Schiff bases were prepared throughout condensation of 1,3-diaminopropane with two different types from Salicyaldehyde and furfuraldehyde. The chemical structures of the prepared Schiff bases were confirmed by using elemental analysis and FT-IR spectra. The synthesized Schiff bases were ...
sch4ureview
... catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical characteristics to that molecule – there are three main components: multiple bo ...
... catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical characteristics to that molecule – there are three main components: multiple bo ...
12 U Chem Review
... catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical characteristics to that molecule – there are three main components: multiple bo ...
... catenation – the property of carbon to form a covalent bond with another carbon atom, forming long chains or rings functional group – a group of atoms in an organic molecule that impart particular physical and chemical characteristics to that molecule – there are three main components: multiple bo ...
Chapter 13 PowerPoint
... mainly product – equilibrium lies to the right A very small K means that the system at equilibrium will consist mainly of reactants – equilibrium position is far to the left The size of K and the time required to reach equilibrium are NOT directly related. Complete sample problem #5. ...
... mainly product – equilibrium lies to the right A very small K means that the system at equilibrium will consist mainly of reactants – equilibrium position is far to the left The size of K and the time required to reach equilibrium are NOT directly related. Complete sample problem #5. ...
Unit 8 Chemical Equilibrium Focusing on Acid
... equation. For each of the following combinations of reagents, predict the product(s), and write a net ionic reaction equation. Balance the equation with simplest integer coefficients, and include physical states for all substances. (a) Copper(II) chloride and potassium carbonate solutions are mixed. ...
... equation. For each of the following combinations of reagents, predict the product(s), and write a net ionic reaction equation. Balance the equation with simplest integer coefficients, and include physical states for all substances. (a) Copper(II) chloride and potassium carbonate solutions are mixed. ...
REACTING MASSES – ACTIVITY SHEET
... a) Calculate the maximum theoretical mass of hydrazine that can be made by reacting 340 g of ammonia with an excess of sodium chlorate. b) In the reaction, only 280 g of hydrazine was produced. Calculate the percentage yield. c) Calculate the atom economy for this way of making hydrazine. 2) Ibuprof ...
... a) Calculate the maximum theoretical mass of hydrazine that can be made by reacting 340 g of ammonia with an excess of sodium chlorate. b) In the reaction, only 280 g of hydrazine was produced. Calculate the percentage yield. c) Calculate the atom economy for this way of making hydrazine. 2) Ibuprof ...
Lectures on Chapter 4, Part 2 Powerpoint 97 Document
... To balance electrons we must put a 4 in front of the Ag, since each oxygen looses two electrons, and they come two per O2! That requires us to put a 4 in front of the silver complex, yielding 8 cyanide ions. 4 Ag(s) + 8 CN -(aq) + O2 (g) 4 Ag(CN)2-(aq) + OH -(aq) We have no hydrogens on the reactant ...
... To balance electrons we must put a 4 in front of the Ag, since each oxygen looses two electrons, and they come two per O2! That requires us to put a 4 in front of the silver complex, yielding 8 cyanide ions. 4 Ag(s) + 8 CN -(aq) + O2 (g) 4 Ag(CN)2-(aq) + OH -(aq) We have no hydrogens on the reactant ...
Chem 150 Unit 2 - Hydrocarbons & Functional Groups
... slightly higher boiling point than alcohols, because they can form two hydrogen bonds with a neighboring molecule (See Figure 4.23 in Raymond) ...
... slightly higher boiling point than alcohols, because they can form two hydrogen bonds with a neighboring molecule (See Figure 4.23 in Raymond) ...