- Kendriya Vidyalaya Jamuna Colliery
... 3. Molarity (M) = It decreases with increase in temperature as volume of solution increases with temperature. 4. Molality (m) = No effect of change of temperature on molality as it is mass to mass ratio. 6. Parts per million (ppm) concentration of very dilute solution is expressed in ppm. Ppm = Vapo ...
... 3. Molarity (M) = It decreases with increase in temperature as volume of solution increases with temperature. 4. Molality (m) = No effect of change of temperature on molality as it is mass to mass ratio. 6. Parts per million (ppm) concentration of very dilute solution is expressed in ppm. Ppm = Vapo ...
Problem 1-2
... The top 15 of the 3rd round are the participants of the 4th round, a oneweek practical training. There are two written five-hour tests - one theoretical and one practical - under the same conditions as at the IChO. Here the team is selected. In this booklet all problems of the selection procedure an ...
... The top 15 of the 3rd round are the participants of the 4th round, a oneweek practical training. There are two written five-hour tests - one theoretical and one practical - under the same conditions as at the IChO. Here the team is selected. In this booklet all problems of the selection procedure an ...
Fall 2006
... a quick check of yourself and find no evidence of splashes or cuts. What should you do next? ...
... a quick check of yourself and find no evidence of splashes or cuts. What should you do next? ...
quantitative chemistry
... physical properties (such as melting point and density) and chemical properties (such as flammability and acidity). The properties of the mixture are similar to those of the components (e.g. a match burns in both air and pure oxygen), though they will vary with its exact composition. The fact that t ...
... physical properties (such as melting point and density) and chemical properties (such as flammability and acidity). The properties of the mixture are similar to those of the components (e.g. a match burns in both air and pure oxygen), though they will vary with its exact composition. The fact that t ...
Unit 7 Reaction Rates and Equilibrium Notes
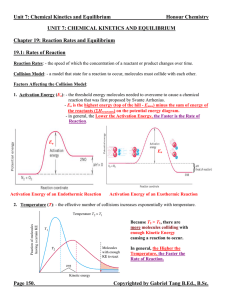
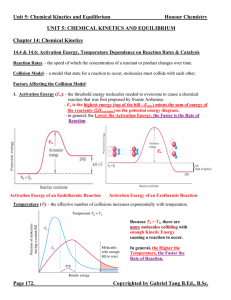
... Equilibrium Position: - the concentrations or pressures of all chemical species at equilibrium state. - depends strongly on the Initial Concentrations of the chemical species. (In contrast, K does NOT depend on initial concentrations, only on temperature and the specific reaction.) - since there all ...
... Equilibrium Position: - the concentrations or pressures of all chemical species at equilibrium state. - depends strongly on the Initial Concentrations of the chemical species. (In contrast, K does NOT depend on initial concentrations, only on temperature and the specific reaction.) - since there all ...
14.1 Dynamic Equilibrium, Keq , and the Mass Action Expression
... Extent of a Reaction Chemical Reaction Most reactions do not occur with 100% conversion to products. At the molecular, when a reaction occurs to form products, some products will back react to form reactants. The extent of the reaction i.e., 20% or 80% can be determine by measuring concentration of ...
... Extent of a Reaction Chemical Reaction Most reactions do not occur with 100% conversion to products. At the molecular, when a reaction occurs to form products, some products will back react to form reactants. The extent of the reaction i.e., 20% or 80% can be determine by measuring concentration of ...
Topic 6 Section C
... (1) When aqueous chlorine is added to sodium bromide solution, the solution becomes orange/brown due to the formation of bromine. Adding an organic solvent to the reaction mixture gives an orange layer. When aqueous chlorine is added to sodium iodide solution, the solution becomes brown due to the f ...
... (1) When aqueous chlorine is added to sodium bromide solution, the solution becomes orange/brown due to the formation of bromine. Adding an organic solvent to the reaction mixture gives an orange layer. When aqueous chlorine is added to sodium iodide solution, the solution becomes brown due to the f ...
Exam 2 Key
... 3. The terms strong electrolyte and weak electrolyte are used in multiple contexts. Discuss how these terms are used in each of the contexts below. Use a maximum of three sentences per context. (8 points) a. When describing a compound: When describing a compound, the term electrolyte refers to the c ...
... 3. The terms strong electrolyte and weak electrolyte are used in multiple contexts. Discuss how these terms are used in each of the contexts below. Use a maximum of three sentences per context. (8 points) a. When describing a compound: When describing a compound, the term electrolyte refers to the c ...
Volumetrie properties of concentrated electrolyte solutions
... New problems arise in the region of concentrated solutions. The dominant factors determining the solution structure in this region are ion-solvent and ion-ion interactions, and the formation of contact ion pairs. It is clear that the relations derived for the region of dilute solutions cannot hold u ...
... New problems arise in the region of concentrated solutions. The dominant factors determining the solution structure in this region are ion-solvent and ion-ion interactions, and the formation of contact ion pairs. It is clear that the relations derived for the region of dilute solutions cannot hold u ...
On the Networking Mechanisms of Additives
... If this was really due only to the accelerating influence of the HCO 30 ion as has been advocated 4,5 (a mechanism for which no evidence has been presented), then the curves of the two materials would be coincident, and they are not. In this regard, it is interesting to note that even when it was tr ...
... If this was really due only to the accelerating influence of the HCO 30 ion as has been advocated 4,5 (a mechanism for which no evidence has been presented), then the curves of the two materials would be coincident, and they are not. In this regard, it is interesting to note that even when it was tr ...
containing complexes of aromatic amino acids
... energies were evaluated directly using the normal-mode frequencies without anharmonic scaling. The local minima associated with each transition structure were identified using the intrinsic reaction coordinates (IRC) method.19 Atomic charges and spin densities have been evaluated using natural popula ...
... energies were evaluated directly using the normal-mode frequencies without anharmonic scaling. The local minima associated with each transition structure were identified using the intrinsic reaction coordinates (IRC) method.19 Atomic charges and spin densities have been evaluated using natural popula ...
Selective Gas-Phase Cleavage at the Peptide
... including them in existing computer-based interpretation of peptide MS/MS spectra. Therefore, thorough investigations for improved understanding, on a molecular level, of the enhancement or absence of certain fragment ions in MS/MS experiments are warranted with the long-term goal of providing addit ...
... including them in existing computer-based interpretation of peptide MS/MS spectra. Therefore, thorough investigations for improved understanding, on a molecular level, of the enhancement or absence of certain fragment ions in MS/MS experiments are warranted with the long-term goal of providing addit ...
Chlorine Chemistry For Water and Waste Treatment
... left end of the curve. The peak of the curve occurs when all of the free ammonia is used up forming chloramines. With excess chlorine due to higher dosages, the chloramines are unstable and destruction occurs due to one or both of the following reactions: 2NH2CI + HOCI -----> N3 + H2O + 3HCI NH3CI + ...
... left end of the curve. The peak of the curve occurs when all of the free ammonia is used up forming chloramines. With excess chlorine due to higher dosages, the chloramines are unstable and destruction occurs due to one or both of the following reactions: 2NH2CI + HOCI -----> N3 + H2O + 3HCI NH3CI + ...
chemistry-resource
... Students’ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
... Students’ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
study material(2014-15) class xii-chemistry
... Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
... Students‘ common errors, un-attempted questions and their remediation. Reviewed Support Materials of the previous year. In order to ensure that the participants come well-prepared for the Workshop, the topics/chapters were distributed among them well in advance. During the Workshop the materials pre ...
CHEM 1412. Chapter 15. Chemical Equilibrium (Homework)
... some reactants and products with reactants slightly favored some reactants and products with products slightly favored essentially all reactants essentially all products ...
... some reactants and products with reactants slightly favored some reactants and products with products slightly favored essentially all reactants essentially all products ...
SQA CfE Higher Chemistry Unit 3: Chemistry in society
... Atom economy • The atom economy measures the proportion of the total mass of all starting materials successfully converted into the desired product. • It can be calculated using the formula shown below in which the masses of products and reactants are those appearing in the balanced equation for the ...
... Atom economy • The atom economy measures the proportion of the total mass of all starting materials successfully converted into the desired product. • It can be calculated using the formula shown below in which the masses of products and reactants are those appearing in the balanced equation for the ...
The reaction pathways of hydrogen peroxide in
... enthalpy, entropy and free energy of the transition states of the formation and breakdown of the intermediate have been calculated. The metal-catalyzed pathway of hydrogen peroxide is dealing with the effect of hydroxyl radicals created by the Fenton reaction and their potential to oxidize the disul ...
... enthalpy, entropy and free energy of the transition states of the formation and breakdown of the intermediate have been calculated. The metal-catalyzed pathway of hydrogen peroxide is dealing with the effect of hydroxyl radicals created by the Fenton reaction and their potential to oxidize the disul ...
(MgCl2 and CaCl2): Osmotic Pressure Calculations
... between a Ca2+ ion and oxygen atoms in a theoretical study of Ca2+ binding with Parvalbumin provided evidence of how the charge in the center of the ion by itself is not sufficient to keep the Ca2+ ion stable in its binding site.34 Another theoretical study of Ca2+ binding in Calbindin reported that a ...
... between a Ca2+ ion and oxygen atoms in a theoretical study of Ca2+ binding with Parvalbumin provided evidence of how the charge in the center of the ion by itself is not sufficient to keep the Ca2+ ion stable in its binding site.34 Another theoretical study of Ca2+ binding in Calbindin reported that a ...