Basic chemistry help is available here for high school or college
... classroom students. All hard copy materials distributed under this exception must have on every page distributed reference to http://www.chemtutor.com as source. Under the same exception granted to classroom teachers, full recognition of Chemtutor must be given when all or any part is included in an ...
... classroom students. All hard copy materials distributed under this exception must have on every page distributed reference to http://www.chemtutor.com as source. Under the same exception granted to classroom teachers, full recognition of Chemtutor must be given when all or any part is included in an ...
Part 1-ICHO-21-25
... This publication contains the competition problems (Volume 2) from the 21st – 40th International Chemistry Olympiads (ICHO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty ICHOs. ...
... This publication contains the competition problems (Volume 2) from the 21st – 40th International Chemistry Olympiads (ICHO) organized in the years 1989 – 2008 and is a continuation of the publication that appeared last year as Volume 1 and contained competition problems from the first twenty ICHOs. ...
Covert Chemical... 2_Couvertures English chimie 4
... Strong Acids and Weak Acids .......................................................................... 3.15 Applications .................................................................................................... 3.18 3.2 THE BASE IONIZATION CONSTANT Kb ..................................... ...
... Strong Acids and Weak Acids .......................................................................... 3.15 Applications .................................................................................................... 3.18 3.2 THE BASE IONIZATION CONSTANT Kb ..................................... ...
1.09 MB / 64 pages
... pairs on the oxygen of ethanol, and another H bond between the –OH group’s hydrogen and a nonbonding electron pair on the oxygen in water. The water molecules have other nonbonding pairs on oxygen and covalently bonded hydrogen, all of which are capable of extending the network of hydrogen bonds. In ...
... pairs on the oxygen of ethanol, and another H bond between the –OH group’s hydrogen and a nonbonding electron pair on the oxygen in water. The water molecules have other nonbonding pairs on oxygen and covalently bonded hydrogen, all of which are capable of extending the network of hydrogen bonds. In ...
document
... Let’s use what we know again We can convert grams of propane to moles of propane 1 mole of propane is 44.09 g of propane So 44.1 g x 1 mole / 44.09 g = 1 mole Know we need 5 moles of oxygen for every mole of propane 1 mol C3H8 x 5 mol O2 / 1 mol C3H8 = 5 mol O2 ...
... Let’s use what we know again We can convert grams of propane to moles of propane 1 mole of propane is 44.09 g of propane So 44.1 g x 1 mole / 44.09 g = 1 mole Know we need 5 moles of oxygen for every mole of propane 1 mol C3H8 x 5 mol O2 / 1 mol C3H8 = 5 mol O2 ...
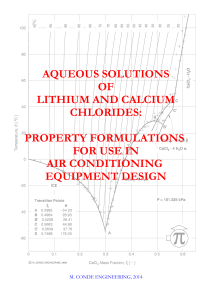
Aqueous solutions of lithium and calcium chlorides
... refrigeration and air conditioning. While they were first studied and characterized as freezing point depressors and used as brines in refrigeration applications, other of their properties, such as the high water affinity, were later recognized as very interesting for the dehydration of gases, in pa ...
... refrigeration and air conditioning. While they were first studied and characterized as freezing point depressors and used as brines in refrigeration applications, other of their properties, such as the high water affinity, were later recognized as very interesting for the dehydration of gases, in pa ...
III. Polyelectrolyte Phenomena (Dautzenberg et al., Polyelectrolytes
... Huggins equation fails at low cs because the primary interactions between chains are electrostatic, not hydrodynamic as this equation assumes; the interpretation is also consistent with the appearance and c dependence of the small angle scattering peak observed for dilute polyelectrolytes under the ...
... Huggins equation fails at low cs because the primary interactions between chains are electrostatic, not hydrodynamic as this equation assumes; the interpretation is also consistent with the appearance and c dependence of the small angle scattering peak observed for dilute polyelectrolytes under the ...
8. Solution Guide to Supplementary Exercises
... ∴ the enthalpy change of combustion of pentan-1-ol is –2 820 kJ mol–1. b) i) The enthalpy change of a reaction depends on the initial and final states of the reaction and is independent of the route by which the reaction may occur. ...
... ∴ the enthalpy change of combustion of pentan-1-ol is –2 820 kJ mol–1. b) i) The enthalpy change of a reaction depends on the initial and final states of the reaction and is independent of the route by which the reaction may occur. ...
Ternary nucleation of inorganic acids, ammonia, and water
... ⫽298.15 K and RH⫽50%. The acid concentration is adjusted so that nucleation rate is 1 cm⫺3 s⫺1 when ammonia concentration is 109 molecules/cm3 . In this figure and for the rest of the paper, the H2 SO4 concentration refers to the total concentration of H2 SO4 共including H2 O–H2 SO4 hydrates兲. Vapors ...
... ⫽298.15 K and RH⫽50%. The acid concentration is adjusted so that nucleation rate is 1 cm⫺3 s⫺1 when ammonia concentration is 109 molecules/cm3 . In this figure and for the rest of the paper, the H2 SO4 concentration refers to the total concentration of H2 SO4 共including H2 O–H2 SO4 hydrates兲. Vapors ...
Concept based notes Chemistry Lab Manual
... Ans. When H2S gas is passed in alkaline medium or in presence of NH4OH, the H+ ions from the dissociation of H2S gas combine with hydroxyl ions (OH-) from the dissociation of NH4OH to from nearly unionised H2O. The removal of H+ ions from the solution causes more H2S to dissociate, thereby increasin ...
... Ans. When H2S gas is passed in alkaline medium or in presence of NH4OH, the H+ ions from the dissociation of H2S gas combine with hydroxyl ions (OH-) from the dissociation of NH4OH to from nearly unionised H2O. The removal of H+ ions from the solution causes more H2S to dissociate, thereby increasin ...
102MSJc14 - Louisiana Tech University
... completion, that is, until one of the reactants runs out. Many reactions do proceed essentially to completion: complete reactions are indicated by . For such reactions it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains ...
... completion, that is, until one of the reactants runs out. Many reactions do proceed essentially to completion: complete reactions are indicated by . For such reactions it can be assumed that the reactants are quantitatively converted to products and that the amount of limiting reactant that remains ...
Chemistry 30 June 2001 Grade 12 Diploma Examination
... A student heated a 120.0 g sample of H2O(l) from 21.0°C to 32.5°C by adding 5.93 kJ of energy. The student then used this data to calculate the specific heat capacity of water and compared it with the standard value. The experimental percentage difference was __________ %. (Record your three-digit a ...
... A student heated a 120.0 g sample of H2O(l) from 21.0°C to 32.5°C by adding 5.93 kJ of energy. The student then used this data to calculate the specific heat capacity of water and compared it with the standard value. The experimental percentage difference was __________ %. (Record your three-digit a ...
Major 01 - KFUPM Faculty List
... Since melting has no change of the chemical composition involved, this is a physical change. B. Its surface turns black when first exposed to air. Color change indicates a chemical reaction. C. When placed in water a gas is formed. Again, formation of a gas indicates a chemical reaction. D. When pla ...
... Since melting has no change of the chemical composition involved, this is a physical change. B. Its surface turns black when first exposed to air. Color change indicates a chemical reaction. C. When placed in water a gas is formed. Again, formation of a gas indicates a chemical reaction. D. When pla ...
Chapter 15: Chemical Equilibrium
... amount of solid present as long as there is some solid present for the system to reach equilibrium. The concentration of a pure solid depends only on the density of the substance, a constant that can be incorporated into the equilibrium constant. 2. Pure liquids and solvents do not appear in equilib ...
... amount of solid present as long as there is some solid present for the system to reach equilibrium. The concentration of a pure solid depends only on the density of the substance, a constant that can be incorporated into the equilibrium constant. 2. Pure liquids and solvents do not appear in equilib ...
HOTS Worksheet
... Ans. (i) Glucose does not give Schiff‘s Test although it contains aldehyde group. (ii) Glucose does not form crystaline product with NaHSO3. Q. 6. B-complex is an often prescribed Vitamin. What is complex about it ? What is its usefulness ? Ans. It is a type of Vitamin which contains B1, B2, B6 and ...
... Ans. (i) Glucose does not give Schiff‘s Test although it contains aldehyde group. (ii) Glucose does not form crystaline product with NaHSO3. Q. 6. B-complex is an often prescribed Vitamin. What is complex about it ? What is its usefulness ? Ans. It is a type of Vitamin which contains B1, B2, B6 and ...