Ch 7New.notebook
... 1,6 diaminohexane is used to make nylon. Analysis of this compound finds its percent composition to be 62.1% C, 13.8% H, and 24.1% N. Its molecular mass is also found to be 116 g/mol. What are the empirical and molecular formulas of this compound? ...
... 1,6 diaminohexane is used to make nylon. Analysis of this compound finds its percent composition to be 62.1% C, 13.8% H, and 24.1% N. Its molecular mass is also found to be 116 g/mol. What are the empirical and molecular formulas of this compound? ...
TRO Chapter 4
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
Chapter 4 Chemical Quantities and Aqueous Reactions
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
Document
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
... dilute solutions have a small amount of solute compared to solvent concentrated solutions have a large amount of solute compared to solvent quantitatively, the relative amount of solute in the solution is called the concentration ...
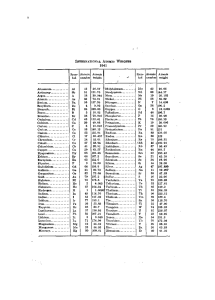
INTEKNATIONAL ATOMIC WEIGHTS Aluminum... Antimony..., Argon
... particularly for more advanced work. It is designed not only to encourage students to undertake special work but to aid them in later years in the solution of practical problems. No claim whatsoever is made for completeness. In their selection of material the authors have been guided simply by their ...
... particularly for more advanced work. It is designed not only to encourage students to undertake special work but to aid them in later years in the solution of practical problems. No claim whatsoever is made for completeness. In their selection of material the authors have been guided simply by their ...
Equilibrium Reversible Reactions
... For example, in a class of 20 students, 10 students could represent sodium ions and 10 students could represent chloride ions. Have 4 sodium ions and 4 chloride ions link arms on the left side of the room to represent sodium chloride particles. Have the remaining 12 students stand on the right side ...
... For example, in a class of 20 students, 10 students could represent sodium ions and 10 students could represent chloride ions. Have 4 sodium ions and 4 chloride ions link arms on the left side of the room to represent sodium chloride particles. Have the remaining 12 students stand on the right side ...
Chapter 18: Chemical Equilibrium
... are not always fast. When carried out under standard conditions, this ammonia-forming reaction is much too slow. To produce ammonia at a rate that is practical, the reaction must be carried out at a much higher temperature than 298 K and a higher pressure than one atmosphere. What happens when one m ...
... are not always fast. When carried out under standard conditions, this ammonia-forming reaction is much too slow. To produce ammonia at a rate that is practical, the reaction must be carried out at a much higher temperature than 298 K and a higher pressure than one atmosphere. What happens when one m ...
chem textbook 2015 - Manitowoc Public School District
... This generally means that your notes are incomplete, meaning that you wrote down much of what was on the board but did not record any of the verbal discussion or rationale used to explain what was taking place. It is important that your notes include your thoughts rather than just what I right on th ...
... This generally means that your notes are incomplete, meaning that you wrote down much of what was on the board but did not record any of the verbal discussion or rationale used to explain what was taking place. It is important that your notes include your thoughts rather than just what I right on th ...
Preparatory Problems of the 40th IChO - IChO-2016
... Watson kept notes about his adventures with Mr. Sherlock Holmes into the 1950s. An interesting, but incomplete story read as follows: ....and was able to spring into a cab and drive to Baker Street, half afraid that I might be too late to assist at the dénouement of the little mystery. I found Sherl ...
... Watson kept notes about his adventures with Mr. Sherlock Holmes into the 1950s. An interesting, but incomplete story read as follows: ....and was able to spring into a cab and drive to Baker Street, half afraid that I might be too late to assist at the dénouement of the little mystery. I found Sherl ...
Chapter 18 pdf
... are not always fast. When carried out under standard conditions, this ammonia-forming reaction is much too slow. To produce ammonia at a rate that is practical, the reaction must be carried out at a much higher temperature than 298 K and a higher pressure than one atmosphere. What happens when one m ...
... are not always fast. When carried out under standard conditions, this ammonia-forming reaction is much too slow. To produce ammonia at a rate that is practical, the reaction must be carried out at a much higher temperature than 298 K and a higher pressure than one atmosphere. What happens when one m ...
kcse chemistry questions
... Write the formula of the compound that would be formed between X and Y. When bromine gas reacts with aqueous sodium hydroxide, the equilibrium represented by the equation: Br2 (aq) +2OH-(aq) Br-(aq) + OBR- (aq) + H2O is established. What observations would be made if a few drops of sulphuric acid we ...
... Write the formula of the compound that would be formed between X and Y. When bromine gas reacts with aqueous sodium hydroxide, the equilibrium represented by the equation: Br2 (aq) +2OH-(aq) Br-(aq) + OBR- (aq) + H2O is established. What observations would be made if a few drops of sulphuric acid we ...
Higher Chemistry Resources Guide - Glow Blogs
... attraction that can operate between all atoms and molecules. These forces are much weaker than all other types of bonding. They are formed as a result of electrostatic attraction between temporary dipoles and induced dipoles caused by movement of electrons in atoms and molecules. The strength of Lon ...
... attraction that can operate between all atoms and molecules. These forces are much weaker than all other types of bonding. They are formed as a result of electrostatic attraction between temporary dipoles and induced dipoles caused by movement of electrons in atoms and molecules. The strength of Lon ...
Higher Chemistry Resources Guide - Glow Blogs
... attraction that can operate between all atoms and molecules. These forces are much weaker than all other types of bonding. They are formed as a result of electrostatic attraction between temporary dipoles and induced dipoles caused by movement of electrons in atoms and molecules. The strength of Lon ...
... attraction that can operate between all atoms and molecules. These forces are much weaker than all other types of bonding. They are formed as a result of electrostatic attraction between temporary dipoles and induced dipoles caused by movement of electrons in atoms and molecules. The strength of Lon ...
chemistry - Textbooks Online
... form a molecule" is required to gain knowledge of the followingi) to know about how atoms of same element form different compounds combining with different elements. ii) to know why particular shapes are adopted by molecules. iii) to understand the specific properties of molecules or ions and the re ...
... form a molecule" is required to gain knowledge of the followingi) to know about how atoms of same element form different compounds combining with different elements. ii) to know why particular shapes are adopted by molecules. iii) to understand the specific properties of molecules or ions and the re ...
indian association of chemistry teachers
... Website : www.careerpointgroup.com, Email: [email protected] ...
... Website : www.careerpointgroup.com, Email: [email protected] ...
Mole Concept - Shailendra Kumar Chemistry
... Calcium metal reacts with hydrochloric acid to yield hydrogen and calcium chloride. Write a balanced chemical equation for the reaction. Determine the volume ...
... Calcium metal reacts with hydrochloric acid to yield hydrogen and calcium chloride. Write a balanced chemical equation for the reaction. Determine the volume ...
Critical Assessment of the Formation of Ionic-Liquid - PATh
... weaker salting-out agents, such as carbohydrates26 or amino acids,27 more hydrophobic, yet water-soluble ionic liquids must be employed. Such ionic liquids are here defined as those fluids composed of long alkyl side chain lengths and/or anions with low hydrogen bond basicity values,8,11 namely [N(CN) ...
... weaker salting-out agents, such as carbohydrates26 or amino acids,27 more hydrophobic, yet water-soluble ionic liquids must be employed. Such ionic liquids are here defined as those fluids composed of long alkyl side chain lengths and/or anions with low hydrogen bond basicity values,8,11 namely [N(CN) ...