ln2_storage_pre
... the critical temperature for both fluids, so you will not get a liquid no matter how much pressure you put on it. The gases in the cylinders are supercritical fluids, though when you get that far above the critical temperature their behavior is much like that of a normal gas unless you get to very h ...
... the critical temperature for both fluids, so you will not get a liquid no matter how much pressure you put on it. The gases in the cylinders are supercritical fluids, though when you get that far above the critical temperature their behavior is much like that of a normal gas unless you get to very h ...
Garnock Academy Level 3 Science Matter Homework 1 SCN 3-05a
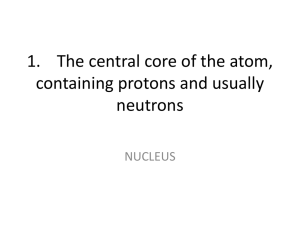
... What is the name given to the atoms and molecules that everything is made up of? ___________________________________________ ...
... What is the name given to the atoms and molecules that everything is made up of? ___________________________________________ ...
Unit 4 Compounds, Naming, Formula Writing
... Molecular Formulas: shows the kinds and numbers of atoms present in a molecule of a compound H2O ...
... Molecular Formulas: shows the kinds and numbers of atoms present in a molecule of a compound H2O ...
ヌミタヌホハ ホヤホミフヒナヘペ メナヌ トホマホツイトイ
... Liquid crystals (LCs) are very sensitive to application of an external electric field due to the large value of dielectric anisotropy, whereas they are practically insensitive to application of magnetic field. About 5 decades ago the idea was born to mix nano-sized magnetic particles (MPs) with nema ...
... Liquid crystals (LCs) are very sensitive to application of an external electric field due to the large value of dielectric anisotropy, whereas they are practically insensitive to application of magnetic field. About 5 decades ago the idea was born to mix nano-sized magnetic particles (MPs) with nema ...
an uncertainty relation for quantum systems with an arbitrarily large
... where the left-hand side is a lower bound of the left-hand side of (2) up to the (Δ/π)2/5 term appearing in the right-hand side of (12). This equation is interesting in many respects. The inequality does not rule out the constancy or the increase of the survival time T with R for large R, i.e., the ...
... where the left-hand side is a lower bound of the left-hand side of (2) up to the (Δ/π)2/5 term appearing in the right-hand side of (12). This equation is interesting in many respects. The inequality does not rule out the constancy or the increase of the survival time T with R for large R, i.e., the ...
N5_Ink_Ex_1_Unit_1
... d) Atoms of boron exist which have the same number of protons but a different number of neutrons from that shown in the table. What name can be used to describe the different atoms of boron? ...
... d) Atoms of boron exist which have the same number of protons but a different number of neutrons from that shown in the table. What name can be used to describe the different atoms of boron? ...
Chapter III: Matter - Norwell Public Schools
... As temperature and pressure change, substances change from one phase to another. ...
... As temperature and pressure change, substances change from one phase to another. ...
“Eggs”periments with Vinegar
... “Eggs”periments with Vinegar Contributed by Elfi Berndl, RocksForKids.com In this activity, students observe chemical reactions as eggshells dissolve in vinegar, and calcium acetate crystals grow. These crystals will look botryoidal (bumpy – like a bunch of grapes or popcorn). Materials ...
... “Eggs”periments with Vinegar Contributed by Elfi Berndl, RocksForKids.com In this activity, students observe chemical reactions as eggshells dissolve in vinegar, and calcium acetate crystals grow. These crystals will look botryoidal (bumpy – like a bunch of grapes or popcorn). Materials ...
Chapter 3 Notes
... i. Phase changes are ALWAYS physical changes. [The composition of the substance does not change, just how close the atoms are to each other and how much ...
... i. Phase changes are ALWAYS physical changes. [The composition of the substance does not change, just how close the atoms are to each other and how much ...
Chemistry 2: matter is made up of atoms
... • Atomic number is the number of protons in the nucleus of an element • Mass number is the sum of protons and neutrons in the nucleus. It is the average of all isotopes of an element • Isotopes are different forms of an element having the same number of protons but different number of neutrons, henc ...
... • Atomic number is the number of protons in the nucleus of an element • Mass number is the sum of protons and neutrons in the nucleus. It is the average of all isotopes of an element • Isotopes are different forms of an element having the same number of protons but different number of neutrons, henc ...
File
... case, since a new substance is formed, it is a chemical change. ***Note that the number and kind of atoms on the left side of the equation equal the number and kind of atoms on the right side of the equation. Only the arrangement of the atoms has changed in going from reactants to products. The equa ...
... case, since a new substance is formed, it is a chemical change. ***Note that the number and kind of atoms on the left side of the equation equal the number and kind of atoms on the right side of the equation. Only the arrangement of the atoms has changed in going from reactants to products. The equa ...
GasLawTheory
... Substiture this into above equation and PM = dRT Note bottom of page 179 on how gas densities differ from liquid densities Hot air balloons and problems associated with ...
... Substiture this into above equation and PM = dRT Note bottom of page 179 on how gas densities differ from liquid densities Hot air balloons and problems associated with ...
Chem Unit 3 Vocabulary
... 16 dynamic condition in which 2 opposing changes occur at equal rates in a closed system 17 pressure exerted by a vapor in equilibrium with its corresponding liquid at a given temperature 18 process by which particles escape from the surface of a non-boiling liquid & enter the gas state 19 substance ...
... 16 dynamic condition in which 2 opposing changes occur at equal rates in a closed system 17 pressure exerted by a vapor in equilibrium with its corresponding liquid at a given temperature 18 process by which particles escape from the surface of a non-boiling liquid & enter the gas state 19 substance ...
practice test2(Answers)
... A) The temperature of steam cannot exceed 100°C. B) The temperature of ice remains at 0°C as it melts. C) The temperature of liquid water increases linearly as it is heated D) The temperature of liquid water remains at 100°C as it boils E) Both liquid water and ice are present at 0°C. ...
... A) The temperature of steam cannot exceed 100°C. B) The temperature of ice remains at 0°C as it melts. C) The temperature of liquid water increases linearly as it is heated D) The temperature of liquid water remains at 100°C as it boils E) Both liquid water and ice are present at 0°C. ...
The Viscoelastic phenomena Viscoelasticity is a general property of
... The glass transition temperature (Tg) is important to polymers as the melting (or freezing) temperature. For example Tg of polystyrene is at approximately 100ºC; therefore it is glassy and brittle at room temperature. In contrast, a rubber whose Tg is at –73ºCis flexible even in the most severe wint ...
... The glass transition temperature (Tg) is important to polymers as the melting (or freezing) temperature. For example Tg of polystyrene is at approximately 100ºC; therefore it is glassy and brittle at room temperature. In contrast, a rubber whose Tg is at –73ºCis flexible even in the most severe wint ...
Chemistry Readings
... The Noble Gases are in Group 18 or Group O of the Periodic Table. They have full outer shells of electrons. They are stable and will not react with other atoms. Atoms that have lost or gained electrons to form ions will have a full outer shell. Stable ions are said to have achieved a Noble Gas elect ...
... The Noble Gases are in Group 18 or Group O of the Periodic Table. They have full outer shells of electrons. They are stable and will not react with other atoms. Atoms that have lost or gained electrons to form ions will have a full outer shell. Stable ions are said to have achieved a Noble Gas elect ...
Camp 1 - Quynh Nguyen Official Website
... • Gas is a fluid form of matter. It fills any container it ...
... • Gas is a fluid form of matter. It fills any container it ...
Abstract
... The critical gas velocity, below which liquid loading occurs, is usually predicted by the Turner criterion [1], which states that liquid loading occurs when the gas is no longer able to drag the largest droplets in the flow upwards. The calculation of the critical velocity therefore requires an esti ...
... The critical gas velocity, below which liquid loading occurs, is usually predicted by the Turner criterion [1], which states that liquid loading occurs when the gas is no longer able to drag the largest droplets in the flow upwards. The calculation of the critical velocity therefore requires an esti ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).