Four Different Kinds of Magnetism
... function of the external field is described by a hysteresis curve. Although this state of aligned domains is not a minimal-energy configuration, it is extremely stable and has been observed to persist for millions of years in seafloor magnetite aligned by the Earth's magnetic field (whose poles can ...
... function of the external field is described by a hysteresis curve. Although this state of aligned domains is not a minimal-energy configuration, it is extremely stable and has been observed to persist for millions of years in seafloor magnetite aligned by the Earth's magnetic field (whose poles can ...
Unit 12 - HKU Physics
... for all gases at low pressures and is referred as the universal gas constant R, where R = 8.31451 J/(mol K). The ideal gas law is given by ...
... for all gases at low pressures and is referred as the universal gas constant R, where R = 8.31451 J/(mol K). The ideal gas law is given by ...
Infrared Spectroscopy
... depends on the separation of two charges (r) and their magnitude (Q). A big dipole moment means 1. large charges separated, or 2. charges separated over a long distance. ...
... depends on the separation of two charges (r) and their magnitude (Q). A big dipole moment means 1. large charges separated, or 2. charges separated over a long distance. ...
Exam 1 - BYU Physics
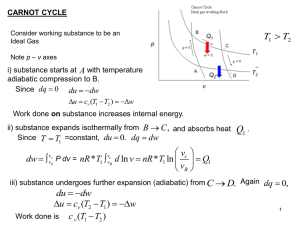
... In which of the three changes is there the largest positive change in entropy? a. AB b. BC c. CA ...
... In which of the three changes is there the largest positive change in entropy? a. AB b. BC c. CA ...
On-surface photo-dissociation of C
... organometallic species. These metallic substituents still show a negative charge when they are free on the surface, but become positive as they bind the tetracene core, see Figure SI2b. As a result, the whole outermost part of the organic molecule is positively charged and one would expect a bigger ...
... organometallic species. These metallic substituents still show a negative charge when they are free on the surface, but become positive as they bind the tetracene core, see Figure SI2b. As a result, the whole outermost part of the organic molecule is positively charged and one would expect a bigger ...
Column Chromatography notes
... • Others include – chitin, derivatives of cellulose, ion exchange resins, etc ...
... • Others include – chitin, derivatives of cellulose, ion exchange resins, etc ...
Intermolecular Forces: Applying What You Know
... Molecules attract each other, and the force of attraction increases rapidly as the intermolecular distance decreases. In a liquid, the molecules are very close to one another and are constantly moving and colliding. When a liquid evaporates, molecules in the liquid must overcome these intermolecular ...
... Molecules attract each other, and the force of attraction increases rapidly as the intermolecular distance decreases. In a liquid, the molecules are very close to one another and are constantly moving and colliding. When a liquid evaporates, molecules in the liquid must overcome these intermolecular ...
SOLIDE STATE Introduction : Crystalline and
... Volatile liquids or soft solids at room temperature and pressure In molecule of ice, 4 molecules of water are attracted by H-bond It gets separated from the hydrides of other elements of the same group because of H bond ...
... Volatile liquids or soft solids at room temperature and pressure In molecule of ice, 4 molecules of water are attracted by H-bond It gets separated from the hydrides of other elements of the same group because of H bond ...
Hypothesis on MATTER
... Region of universal medium, about a 3D matter-body, store work in the form of distortions (and energy in the form of stress due to distortions) to sustain integrity and stability of 3D matterbodies and its state (of constant motion). This region of universal medium is its matter-field. A gap in univ ...
... Region of universal medium, about a 3D matter-body, store work in the form of distortions (and energy in the form of stress due to distortions) to sustain integrity and stability of 3D matterbodies and its state (of constant motion). This region of universal medium is its matter-field. A gap in univ ...
COLLECT DATA TAKS QUESTIONS SPRING 2003 – 10: (7) Which
... Louis Pasteur’s Experiment Like many other scientific breakthroughs, the discovery of immunization happened by accident. In 1880 Louis Pasteur was trying to protect chickens from cholera. To study the disease, Pasteur and his assistants gave injections of cholera bacteria to several groups of chicke ...
... Louis Pasteur’s Experiment Like many other scientific breakthroughs, the discovery of immunization happened by accident. In 1880 Louis Pasteur was trying to protect chickens from cholera. To study the disease, Pasteur and his assistants gave injections of cholera bacteria to several groups of chicke ...
Class 1
... „placing confidence‟ on its use in relation to the expression for thermal conductivity, where there is no electric field applied. Why is this so? The justification for this can be thought of as follows: Electrons are moving very rapidly, and in a random manner as a result of their thermal energy. Th ...
... „placing confidence‟ on its use in relation to the expression for thermal conductivity, where there is no electric field applied. Why is this so? The justification for this can be thought of as follows: Electrons are moving very rapidly, and in a random manner as a result of their thermal energy. Th ...
A. Sate of the art
... pulses and can be well localized. For instance, the advantages of laser-surgery rely on those properties. Still, energy absorption mechanisms in laser-matter interaction are not well known, this is specifically the case for large-scale targets (several nm). In the last years, simulations of the inte ...
... pulses and can be well localized. For instance, the advantages of laser-surgery rely on those properties. Still, energy absorption mechanisms in laser-matter interaction are not well known, this is specifically the case for large-scale targets (several nm). In the last years, simulations of the inte ...
Chapter 1
... boundaries. Note: This definition outlines the key topics in the study of fluids: (1) fluid statics (fluids at rest), (2) momentum and energy analyses (fluids in motion), and (3) viscous effects and all sections considering pressure forces (effects of fluids on boundaries). Fluid - A substance which ...
... boundaries. Note: This definition outlines the key topics in the study of fluids: (1) fluid statics (fluids at rest), (2) momentum and energy analyses (fluids in motion), and (3) viscous effects and all sections considering pressure forces (effects of fluids on boundaries). Fluid - A substance which ...
Chapter 1 Glossary The Nature of Chemistry
... A reaction in which one of the products is insoluble in water and comes out of solution as a solid. Precipitate A solid that comes out of solution. Precipitation The process of forming a solid in a solution. Crystals Solid particles whose component atoms, ions, or molecules are arranged in an organ ...
... A reaction in which one of the products is insoluble in water and comes out of solution as a solid. Precipitate A solid that comes out of solution. Precipitation The process of forming a solid in a solution. Crystals Solid particles whose component atoms, ions, or molecules are arranged in an organ ...
Chapter 9: Chemical Bonding I: Lewis Theory
... 1) Ionic Bonding A) Ionic Bonding results from electron transfer. B) Occurs between metals & nonmetals. i) Metals lose electrons to form cations while nonmetals gain electrons to form anions. C) Ion pair is more stable than separated ions. D) Found as a 3-D crystal lattices containing alternating ca ...
... 1) Ionic Bonding A) Ionic Bonding results from electron transfer. B) Occurs between metals & nonmetals. i) Metals lose electrons to form cations while nonmetals gain electrons to form anions. C) Ion pair is more stable than separated ions. D) Found as a 3-D crystal lattices containing alternating ca ...
Chemical Thermodynamics John Murrell Introduction
... energies that arise in thermodynamics; for this discussion we will ignore the difference between U and H, which we have seen is generally small. For a low pressure gas we can multiply the molecular energy by Avogadro’s constant, 6.022x1023 mol-1, to get energy per mole, and this will be good enough ...
... energies that arise in thermodynamics; for this discussion we will ignore the difference between U and H, which we have seen is generally small. For a low pressure gas we can multiply the molecular energy by Avogadro’s constant, 6.022x1023 mol-1, to get energy per mole, and this will be good enough ...
MACROSCOPIC QUANTUM PHENOMENA FROM PAIRING IN SUPERCONDUCTORS
... to unity at 7 = 0, where “all of the electrons” are in the superfluid condensate. A second important theoretical advance came in the following year, when Fritz and Hans London set down their phenomenological theory of the electromagnetic properties of superconductors, in which the diamagnetic rather ...
... to unity at 7 = 0, where “all of the electrons” are in the superfluid condensate. A second important theoretical advance came in the following year, when Fritz and Hans London set down their phenomenological theory of the electromagnetic properties of superconductors, in which the diamagnetic rather ...
click - Chemsheets
... Substances separated according to relative affinity (attraction) to stationary and mobile phase. If stronger affinity for mobile phase, then move quickly If stronger affinity for stationary phase, the move slowly ...
... Substances separated according to relative affinity (attraction) to stationary and mobile phase. If stronger affinity for mobile phase, then move quickly If stronger affinity for stationary phase, the move slowly ...
Principles of Electrostatic Chucks
... and processing conditions. If a Si wafer resting on a 25°C rf target is exposed to plasma power, the initial rate of temperature rise will be controlled by wafer heat capacity, and the maximum attainable temperature will be set by the above radiation formula. This ignores radation from the plasma an ...
... and processing conditions. If a Si wafer resting on a 25°C rf target is exposed to plasma power, the initial rate of temperature rise will be controlled by wafer heat capacity, and the maximum attainable temperature will be set by the above radiation formula. This ignores radation from the plasma an ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).