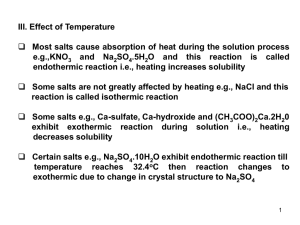
Heating of a Confined Plasma by Oscillating Electromagnetic Fields
... distribution conditions (15) may be violated. Therefore, Eq. (11) should properly be regarded only as an estimate of the flux. There is one further effect which a more sophisticated treatment of transit-time heating should take into account ; namely, that in the analogous situation of mechanical par ...
... distribution conditions (15) may be violated. Therefore, Eq. (11) should properly be regarded only as an estimate of the flux. There is one further effect which a more sophisticated treatment of transit-time heating should take into account ; namely, that in the analogous situation of mechanical par ...
Measuring Temperature: A thermometer measures temperature
... wet cloth over the bulb of a mercury thermometer and then blowing air over the cloth until the water evaporates. Since evaporation takes up heat, the thermometer will cool to a lower temperature than a thermometer with a dry bulb at the same time and place. Wet bulb temperatures can be used along wi ...
... wet cloth over the bulb of a mercury thermometer and then blowing air over the cloth until the water evaporates. Since evaporation takes up heat, the thermometer will cool to a lower temperature than a thermometer with a dry bulb at the same time and place. Wet bulb temperatures can be used along wi ...
CSUS Department of Chemistry Molecular Shapes Chem. 1A Page
... ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in structure 7 has a formal charge, but in structure 8 the oxygen atom has a −1 formal charge and the double‐bonded chlorine atom has a +1 formal charge. In most cases, structures without formal charges are more stable, so we pre ...
... ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in structure 7 has a formal charge, but in structure 8 the oxygen atom has a −1 formal charge and the double‐bonded chlorine atom has a +1 formal charge. In most cases, structures without formal charges are more stable, so we pre ...
Document
... 5.(a) Thermal conductivity is the rate of flow of heat per second normal to 1m2 of a material when the temperature gradient is 1 K m-1. (b) (i) in solids the atoms are closely packed and have strong intermolecular forces as they vibrate within a fixed lattice. When one end is heated, the atoms vibra ...
... 5.(a) Thermal conductivity is the rate of flow of heat per second normal to 1m2 of a material when the temperature gradient is 1 K m-1. (b) (i) in solids the atoms are closely packed and have strong intermolecular forces as they vibrate within a fixed lattice. When one end is heated, the atoms vibra ...
Name: Moles Convert 26.33 g Si to moles. Convert 3.00 mol Sn to
... Measure the mass of the sample of Silicon (Si). How many Si atoms are in your sample? How many moles are in the sample of aluminum foil? Weigh out 0.20 mol of NaCl (salt). I placed ______g of sugar in ______mL of water. What is the concentration of the solution? This is measured in Molarity (M). 5. ...
... Measure the mass of the sample of Silicon (Si). How many Si atoms are in your sample? How many moles are in the sample of aluminum foil? Weigh out 0.20 mol of NaCl (salt). I placed ______g of sugar in ______mL of water. What is the concentration of the solution? This is measured in Molarity (M). 5. ...
Manufacturing Processes - Philadelphia University Jordan
... Fig. 2-8: Hydrogen bonding (water). ...
... Fig. 2-8: Hydrogen bonding (water). ...
What Is Magnetism?
... 2. Circle the letter of the mineral in rocks that is magnetic. a. magnesia b. polaris c. magnetite d. iron 3. The attraction or repulsion of magnetic materials is called ________________________. 4. Is the following sentence true or false? Magnetic rocks are known as lodestones. ____________________ ...
... 2. Circle the letter of the mineral in rocks that is magnetic. a. magnesia b. polaris c. magnetite d. iron 3. The attraction or repulsion of magnetic materials is called ________________________. 4. Is the following sentence true or false? Magnetic rocks are known as lodestones. ____________________ ...
Chapter 8: Magnetic and Electrical Properties 1
... •Ions on the octahedral sites interact with those on the tetrahedral site, but in this case interact through the oxide ions and the spins align antiparallel (like NiO). •In AFe2O4, Fe3+ on tetrahedral sites are aligned antiparallel to those on octahedral sites, so there is no net magnetization from ...
... •Ions on the octahedral sites interact with those on the tetrahedral site, but in this case interact through the oxide ions and the spins align antiparallel (like NiO). •In AFe2O4, Fe3+ on tetrahedral sites are aligned antiparallel to those on octahedral sites, so there is no net magnetization from ...
Chem fundamentals 1a
... Single lines = single bonds = 2 electrons Double lines = double bonds = 4 electrons Nonbonding electrons (depends on the element) are not shown but are counted to balance electrons. O has 4 nonbonding electrons; N has 2. Organic chemical structures often are written to omit the H atoms, wh ...
... Single lines = single bonds = 2 electrons Double lines = double bonds = 4 electrons Nonbonding electrons (depends on the element) are not shown but are counted to balance electrons. O has 4 nonbonding electrons; N has 2. Organic chemical structures often are written to omit the H atoms, wh ...
The heat of combustion of caffeine was determined by first burning be
... The enthalpy change is ∆Hvap = 40 kJ/mol when water boils at 100 ◦ C and 1 atm. This accounts for both the increase in internal energy as the molecules go from the liquid to vapour phase, as well as the expansion of the volume when water vapor forms. What fraction of the enthalpy change is due to th ...
... The enthalpy change is ∆Hvap = 40 kJ/mol when water boils at 100 ◦ C and 1 atm. This accounts for both the increase in internal energy as the molecules go from the liquid to vapour phase, as well as the expansion of the volume when water vapor forms. What fraction of the enthalpy change is due to th ...
S. Savin
... (~ 90 km) is transparent for ions with larger gyroradius, which transfer both parallel and perpendicular momentum and acquire the cross-current potential. The TCS is driven by the Hall current, generated by a part of the surface charge current at the TCS dF ~300 V ...
... (~ 90 km) is transparent for ions with larger gyroradius, which transfer both parallel and perpendicular momentum and acquire the cross-current potential. The TCS is driven by the Hall current, generated by a part of the surface charge current at the TCS dF ~300 V ...
Chem. 1A Week 11 Discussion Notes Dr. Mack/S12 Page 1 of 5 B
... None of the atoms in structures 5 or 6 has a formal charge, so these two structures are predicted to be stable. In this case, structures 5 and 6 are said to be “isomers,” both with the molecular formula C2H6O. (Structure 5 is ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in ...
... None of the atoms in structures 5 or 6 has a formal charge, so these two structures are predicted to be stable. In this case, structures 5 and 6 are said to be “isomers,” both with the molecular formula C2H6O. (Structure 5 is ethyl alcohol and structure 6 is dimethyl ether.) None of the atoms in ...
n - UCL Department of Electronic and Electrical Engineering
... solid structures using the sol-gel process to give silicates with the same structure as the liquid crystal phase. • Other methods can be used to form metal nanoparticles ...
... solid structures using the sol-gel process to give silicates with the same structure as the liquid crystal phase. • Other methods can be used to form metal nanoparticles ...
CASE STUDY 1
... • Does not resolve compounds well, but is a good starting point for analysis, similar to the colourimetric tests described earlier. • Requires compounds to be visible, either by visible light emission or by ultraviolet light absorptivity. • May also be used for MALDI mass spectrometry, although this ...
... • Does not resolve compounds well, but is a good starting point for analysis, similar to the colourimetric tests described earlier. • Requires compounds to be visible, either by visible light emission or by ultraviolet light absorptivity. • May also be used for MALDI mass spectrometry, although this ...
Plasma Oscillations
... material in the Universe exists in the plasma state, even though Saha eqn may not be applicable to some of this plasma. ...
... material in the Universe exists in the plasma state, even though Saha eqn may not be applicable to some of this plasma. ...
Climate and Weather of the Sun-Earth System (CAWSES): Selected Papers... Edited by T. Tsuda, R. Fujii, K. Shibata, and M....
... anisotropies observed in the solar wind at the orbit of Earth are muted by scattering of some sort after leaving the Sun. The principal expansion of the wind is transverse to the radial expansion, and the approximately radial magnetic field, once the wind is up to speed, so a wind without particle s ...
... anisotropies observed in the solar wind at the orbit of Earth are muted by scattering of some sort after leaving the Sun. The principal expansion of the wind is transverse to the radial expansion, and the approximately radial magnetic field, once the wind is up to speed, so a wind without particle s ...
Week 4 AB - Help-A-Bull
... thermal conductivity … … they pick up the thermal energy from vibrating atoms and transfer to atoms elsewhere … It is easy to think of Thermal Conductivity the same way we think of Electrical Conductivity … ...
... thermal conductivity … … they pick up the thermal energy from vibrating atoms and transfer to atoms elsewhere … It is easy to think of Thermal Conductivity the same way we think of Electrical Conductivity … ...
Analysis of PEA photopolymers at zero spatial frequency limit
... type of binder, since this component determines to a great extent the choice of monomer, dye and initiator used in the photopolymer. Analyzing the behavior of a photopolymer as an optic storage medium is a complicated task. Normally, these materials are used in holographic applications, where high v ...
... type of binder, since this component determines to a great extent the choice of monomer, dye and initiator used in the photopolymer. Analyzing the behavior of a photopolymer as an optic storage medium is a complicated task. Normally, these materials are used in holographic applications, where high v ...
Atomic Bonding - New Academic Science
... of distribution of electrons is spherical around the nucleus and it is possible to draw a spherical boundary surface, inside which there is a 95% possibility of finding the electron. The electron has a fixed energy and a fixed spatial distribution called an orbital. The electron have the same spatia ...
... of distribution of electrons is spherical around the nucleus and it is possible to draw a spherical boundary surface, inside which there is a 95% possibility of finding the electron. The electron has a fixed energy and a fixed spatial distribution called an orbital. The electron have the same spatia ...
State of matter

In physics, a state of matter is one of the distinct forms that matter takes on. Four states of matter are observable in everyday life: solid, liquid, gas, and plasma. Many other states are known, such as Bose–Einstein condensates and neutron-degenerate matter, but these only occur in extreme situations such as ultra cold or ultra dense matter. Other states, such as quark–gluon plasmas, are believed to be possible but remain theoretical for now. For a complete list of all exotic states of matter, see the list of states of matter.Historically, the distinction is made based on qualitative differences in properties. Matter in the solid state maintains a fixed volume and shape, with component particles (atoms, molecules or ions) close together and fixed into place. Matter in the liquid state maintains a fixed volume, but has a variable shape that adapts to fit its container. Its particles are still close together but move freely. Matter in the gaseous state has both variable volume and shape, adapting both to fit its container. Its particles are neither close together nor fixed in place. Matter in the plasma state has variable volume and shape, but as well as neutral atoms, it contains a significant number of ions and electrons, both of which can move around freely. Plasma is the most common form of visible matter in the universe.The term phase is sometimes used as a synonym for state of matter, but a system can contain several immiscible phases of the same state of matter (see Phase (matter) for more discussion of the difference between the two terms).