Gelatinization of Starch
... enantiomer is one of two stereoisomers that are mirror images of each other, non-superposable. ...
... enantiomer is one of two stereoisomers that are mirror images of each other, non-superposable. ...
Microbial fermentation (Enzymology,metabolic pathways and
... Enzymes. Enzymes equilibrium state. Factors effects enzymes catalytic activity. Natural mechanisms for regulating enzyme activity. Enzymes activators. Classification of enzymes. ...
... Enzymes. Enzymes equilibrium state. Factors effects enzymes catalytic activity. Natural mechanisms for regulating enzyme activity. Enzymes activators. Classification of enzymes. ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... (5 x 1 = 5 marks) (11) Ethanol acts as a ________ inhibitor of alcohol dehydrogenase when used in methanol poisoning. (12) ________ is a naturally occurring ribozyme. (13) Stability of an enzyme can be improved by introducing _______ bonds in the enzyme structure. (14) ________ enzyme is used in the ...
... (5 x 1 = 5 marks) (11) Ethanol acts as a ________ inhibitor of alcohol dehydrogenase when used in methanol poisoning. (12) ________ is a naturally occurring ribozyme. (13) Stability of an enzyme can be improved by introducing _______ bonds in the enzyme structure. (14) ________ enzyme is used in the ...
Enzymes
... •Each enzyme binds to a single type of substrate > both have complementary structure •substrate overall shape and charge distribution allow it to enter and interact with the enzymes active site. E + S > ES > E+ P ...
... •Each enzyme binds to a single type of substrate > both have complementary structure •substrate overall shape and charge distribution allow it to enter and interact with the enzymes active site. E + S > ES > E+ P ...
Relationship between Protein Synthesis and Secretion in Liver Cells
... The effect of rotenone, 2,4-dinitrophenol, fructose and glycerol on the release into the medium of lactate dehydrogenase and glutamate dehydrogenase, enzymes of the cytoplasm and mitochondria, respectively, was assayed. Enzyme activities were monitored in both total cell suspension and incubation me ...
... The effect of rotenone, 2,4-dinitrophenol, fructose and glycerol on the release into the medium of lactate dehydrogenase and glutamate dehydrogenase, enzymes of the cytoplasm and mitochondria, respectively, was assayed. Enzyme activities were monitored in both total cell suspension and incubation me ...
free energy - HCC Learning Web
... • ATP (adenosine triphosphate) is the cell’s energy shuttle • ATP is composed of ribose (a sugar), adenine (a purine- nitrogenous base), and three phosphate groups • The bonds between the phosphate groups on ATP can be broken by hydrolysis. • When the terminal phosphate bond is broken, a molecule of ...
... • ATP (adenosine triphosphate) is the cell’s energy shuttle • ATP is composed of ribose (a sugar), adenine (a purine- nitrogenous base), and three phosphate groups • The bonds between the phosphate groups on ATP can be broken by hydrolysis. • When the terminal phosphate bond is broken, a molecule of ...
Slide 1
... reliance on anaerobic glycolysis. Remember that H+ ions are produced during glycolysis which are buffered by pyruvate which reduces to lactate. • The rate controlling step of glycolysis is catalyzed by an enzyme called phosphofructose kinase (PFK). PCr levels exert some control over the activity of ...
... reliance on anaerobic glycolysis. Remember that H+ ions are produced during glycolysis which are buffered by pyruvate which reduces to lactate. • The rate controlling step of glycolysis is catalyzed by an enzyme called phosphofructose kinase (PFK). PCr levels exert some control over the activity of ...
BIO 16l EXAM2 SUMMER6WKKey
... a. depends on unusual amino acids not common in proteins. b. has a certaifi unique amino acid to fit each substrate. C. is shaped to fit a certain substrate molecule. d. is lined with glycolipids and glycoproteins. e. passes electrons from one part ofthe substrate to another. ...
... a. depends on unusual amino acids not common in proteins. b. has a certaifi unique amino acid to fit each substrate. C. is shaped to fit a certain substrate molecule. d. is lined with glycolipids and glycoproteins. e. passes electrons from one part ofthe substrate to another. ...
INBORN ERRORS OF AMINO ACIDS METABOLISM
... Tyrosinemia is an extremely rare but treatable hereditary disorder. When the body cannot break down tyrosine, high levels build up in the blood and form a toxic substance (known as succinylacetone) in the liver, kidneys, and central nervous system. This means that if tyrosinemia isn't treated, it m ...
... Tyrosinemia is an extremely rare but treatable hereditary disorder. When the body cannot break down tyrosine, high levels build up in the blood and form a toxic substance (known as succinylacetone) in the liver, kidneys, and central nervous system. This means that if tyrosinemia isn't treated, it m ...
Summary of fatty acid synthesis
... 1. Humans do not have the enzymes required to introduce double bonds past the number 9 carbon of fatty acids. 2. Therefore, linoleic and linolenic acids, both important precursor molecules, are considered essential fatty acids ...
... 1. Humans do not have the enzymes required to introduce double bonds past the number 9 carbon of fatty acids. 2. Therefore, linoleic and linolenic acids, both important precursor molecules, are considered essential fatty acids ...
Amino acids degradation and synthesis
... Ketone bodies Ketone bodies are three water-soluble compounds that are produced as by-products when fatty acids are broken down for energy in the liver and kidney. The three ketone bodies are acetone, acetoacetic acid and beta-hydroxybutyric acid. Ketone bodies are transported from the liver to oth ...
... Ketone bodies Ketone bodies are three water-soluble compounds that are produced as by-products when fatty acids are broken down for energy in the liver and kidney. The three ketone bodies are acetone, acetoacetic acid and beta-hydroxybutyric acid. Ketone bodies are transported from the liver to oth ...
Study of the distribution of autotrophic CO2 fixation
... dehydratase. This [4Fe–4S] and FAD-containing dehydratase (Martins et al., 2004; Buckel & Golding, 2006) is considered a key enzyme of the 4-hydroxybutyrate part of both cycles. Its product, crotonyl-CoA, is further converted to acetoacetyl-CoA and then to two acetyl-CoA molecules, closing the cycle ...
... dehydratase. This [4Fe–4S] and FAD-containing dehydratase (Martins et al., 2004; Buckel & Golding, 2006) is considered a key enzyme of the 4-hydroxybutyrate part of both cycles. Its product, crotonyl-CoA, is further converted to acetoacetyl-CoA and then to two acetyl-CoA molecules, closing the cycle ...
Magnesium: Mineral Link to Energy
... Most sports are of an interval nature, and they rely on the immediate and nonoxidative energy systems heavily. Hoever, even in short duration exercise, some oxidate energy may be involved. ...
... Most sports are of an interval nature, and they rely on the immediate and nonoxidative energy systems heavily. Hoever, even in short duration exercise, some oxidate energy may be involved. ...
Plant and Soil
... uptake system is repressed by organic acids. Studies with the organic acids utilization mutants will show some evidence supporting the repression hypothesis. Cells grown on any substrate had glucosedependent 02 consumption, which support the previous observation indicating the presence of a constitu ...
... uptake system is repressed by organic acids. Studies with the organic acids utilization mutants will show some evidence supporting the repression hypothesis. Cells grown on any substrate had glucosedependent 02 consumption, which support the previous observation indicating the presence of a constitu ...
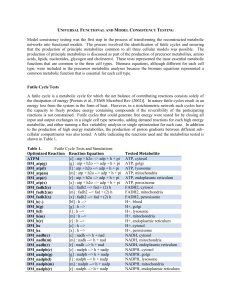
Universal Functional and Model Consistency Testing
... each of these fatty acids could be catabolized to produce energy, the influx of all other carbon sources including glucose was constrained to zero and internal demand for cytosolic ATP was maximized. The myocyte simulations demonstrated that a unit of proton per fatty acid was required to balance fa ...
... each of these fatty acids could be catabolized to produce energy, the influx of all other carbon sources including glucose was constrained to zero and internal demand for cytosolic ATP was maximized. The myocyte simulations demonstrated that a unit of proton per fatty acid was required to balance fa ...
Appendix B HISS Codes for Metabolic Investigations
... early medical management. A dialogue with the department is encouraged and may expedite more complex investigations. General laboratory requirements are covered in SOP_GEN_003 Notes for guidance of staff using the biochemical services (non-metabolic investigations). This includes general information ...
... early medical management. A dialogue with the department is encouraged and may expedite more complex investigations. General laboratory requirements are covered in SOP_GEN_003 Notes for guidance of staff using the biochemical services (non-metabolic investigations). This includes general information ...
PYRIMIDINE METABOLISM
... After removal of the phosphates by various phosphatases, the nucleosides are cleaved to the base by the same nucleoside phosphorylase that catalyzes the salvage reaction. The equilibrium constant for this reaction is near 1, so that it can go in either direction depending on the relative levels of t ...
... After removal of the phosphates by various phosphatases, the nucleosides are cleaved to the base by the same nucleoside phosphorylase that catalyzes the salvage reaction. The equilibrium constant for this reaction is near 1, so that it can go in either direction depending on the relative levels of t ...
Metabolic acidosis
... • Normal daily production of lactate 15 to 30 mmol/kg per day • All of this lactic acid is converted to CO2 and water with no net acid-base effect ...
... • Normal daily production of lactate 15 to 30 mmol/kg per day • All of this lactic acid is converted to CO2 and water with no net acid-base effect ...
9-1 PowerPoint
... Cellular respiration is the process that releases energy form food in the presence of oxygen. Overall respiration - sugars + oxygen carbon dioxide + water. (C6H12O6 + 6O2 6CO2 + 6 H2O Cellular respiration involves a series of controlled reactions that slowly release the energy stored in food. Th ...
... Cellular respiration is the process that releases energy form food in the presence of oxygen. Overall respiration - sugars + oxygen carbon dioxide + water. (C6H12O6 + 6O2 6CO2 + 6 H2O Cellular respiration involves a series of controlled reactions that slowly release the energy stored in food. Th ...
VCE Biology TSFX REVISION LECTURE UNIT 3 Part 1
... Students are expected to understand that polypeptides and proteins are polymers of amino acids, formed through condensation reactions. They are also expected to understand that the primary structure of a polypeptide or protein is the sequence of amino acids that form the polypeptide or protein, and ...
... Students are expected to understand that polypeptides and proteins are polymers of amino acids, formed through condensation reactions. They are also expected to understand that the primary structure of a polypeptide or protein is the sequence of amino acids that form the polypeptide or protein, and ...
Chapter 12 - The Citric Acid Cycle Energy in the citric acid cycle
... (other components are dissolved in the matrix) • Dehydrogenation is stereospecific; only the trans isomer is formed • Substrate analog malonate is a competitive inhibitor of the SDH complex ...
... (other components are dissolved in the matrix) • Dehydrogenation is stereospecific; only the trans isomer is formed • Substrate analog malonate is a competitive inhibitor of the SDH complex ...
sheet#30
... citrulline which leaves the mitochondria. Now citrulline carries two out of the three groups that are present in urea. The third group which is the second amino group is taken from aspartate. 6|Page ...
... citrulline which leaves the mitochondria. Now citrulline carries two out of the three groups that are present in urea. The third group which is the second amino group is taken from aspartate. 6|Page ...
0 13C labeling of the tricarboxylic acid cycle and carbon conversion
... hydroxy fatty acids is ricinoleic acid from the castor plant (Ricinus communis), which also produces the highly toxic compound ricin that has eliminated castor plant cultivation in the United States. Lesquerella produces lesquerolic acid, a hydroxy fatty acid with only two additional carbons as comp ...
... hydroxy fatty acids is ricinoleic acid from the castor plant (Ricinus communis), which also produces the highly toxic compound ricin that has eliminated castor plant cultivation in the United States. Lesquerella produces lesquerolic acid, a hydroxy fatty acid with only two additional carbons as comp ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑