Student notes in ppt
... Ketogenesis occurs when glycogen stores are depleted such as during fasting and in undiagnosed diabetics Diabetes is a metabolic form of carbohydrate "starvation," and characterized by elevated concentrations of acetoacetate and D-hydroxybutyrate in the blood and urine. Diabetics can have high lev ...
... Ketogenesis occurs when glycogen stores are depleted such as during fasting and in undiagnosed diabetics Diabetes is a metabolic form of carbohydrate "starvation," and characterized by elevated concentrations of acetoacetate and D-hydroxybutyrate in the blood and urine. Diabetics can have high lev ...
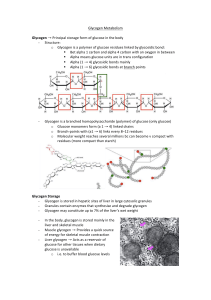
Glycogen!Metabolism! ! Glycogen$→!Principal!storage!form!of
... Fates$of$GlucoseI6IPhosphate$$ - In!skeletal!muscle!G6P!may!enter!glycolysis!and!serve!as!an!energy!source!! - In!liver!G6P!may!be!dephosphorylated!(to!glucose)!for!release!into!the!blood!to!be! transported! o Reaction!catalysed!by!Glucose=6=phosphatase! - Glucose=6=Phosphatase!catalyses!the!followi ...
... Fates$of$GlucoseI6IPhosphate$$ - In!skeletal!muscle!G6P!may!enter!glycolysis!and!serve!as!an!energy!source!! - In!liver!G6P!may!be!dephosphorylated!(to!glucose)!for!release!into!the!blood!to!be! transported! o Reaction!catalysed!by!Glucose=6=phosphatase! - Glucose=6=Phosphatase!catalyses!the!followi ...
CHE 312 - UB`s Department of Chemistry
... Thioesters and their role in metabolism: acetyl CoA Vitamins in metabolism: B, C, D, E, K Identify isomerization, ligation, oxidation-reduction, group transfer, hydrolytic cleavage and lyase reactions Assessment: Chapter 15 homework & Midterm 4 Chapter 16 – Glycolysis (time permitting) Stage 1 of gl ...
... Thioesters and their role in metabolism: acetyl CoA Vitamins in metabolism: B, C, D, E, K Identify isomerization, ligation, oxidation-reduction, group transfer, hydrolytic cleavage and lyase reactions Assessment: Chapter 15 homework & Midterm 4 Chapter 16 – Glycolysis (time permitting) Stage 1 of gl ...
Full Text PDF - Mary Ann Liebert, Inc. publishers
... ples that were used in Figure 1A, both wild type and Abcd1 - . Results are shown for the Abcd1 - membrane (Fig. 1B). We also quantified mRNA and protein expression levels and found that pyruvate kinase is repressed in 12 month-old Abcd1 - spinal cord (Fig. 1C, D). The expression of the four other ox ...
... ples that were used in Figure 1A, both wild type and Abcd1 - . Results are shown for the Abcd1 - membrane (Fig. 1B). We also quantified mRNA and protein expression levels and found that pyruvate kinase is repressed in 12 month-old Abcd1 - spinal cord (Fig. 1C, D). The expression of the four other ox ...
Multiple Choice
... A. prostaglandins B. thromboxanes C. leukotrienes D. isoprenoids E. All of the above are derived from arachidonic acid. 15. Transmembrane lipid asymmetry is maintained in most cells by the action of: A. a phospholipid-specific flippase B. bacteriorhodopsin C. Na+,K+-ATPase D. G proteins E. protein k ...
... A. prostaglandins B. thromboxanes C. leukotrienes D. isoprenoids E. All of the above are derived from arachidonic acid. 15. Transmembrane lipid asymmetry is maintained in most cells by the action of: A. a phospholipid-specific flippase B. bacteriorhodopsin C. Na+,K+-ATPase D. G proteins E. protein k ...
Chemistry - cloudfront.net
... Four of carbon’s six electrons are available to form bonds with other atoms. Thus, you will always see four lines connecting a carbon atom to other atoms, each line representing a pair of shared electrons (one electron from carbon and one from another atom). Complex molecules can be formed by string ...
... Four of carbon’s six electrons are available to form bonds with other atoms. Thus, you will always see four lines connecting a carbon atom to other atoms, each line representing a pair of shared electrons (one electron from carbon and one from another atom). Complex molecules can be formed by string ...
REVISION FOR ENERGY
... The hydrogen electron (e‾) splits from the hydrogen atom and passes down the ETC This provides sufficient energy to re-synthesise 34 ATP molecules The hydrogen ion (H+) combines with oxygen to form water (H2O) ...
... The hydrogen electron (e‾) splits from the hydrogen atom and passes down the ETC This provides sufficient energy to re-synthesise 34 ATP molecules The hydrogen ion (H+) combines with oxygen to form water (H2O) ...
ENERGY
... The hydrogen electron (e‾) splits from the hydrogen atom and passes down the ETC This provides sufficient energy to re-synthesise 34 ATP molecules The hydrogen ion (H+) combines with oxygen to form water (H2O) ...
... The hydrogen electron (e‾) splits from the hydrogen atom and passes down the ETC This provides sufficient energy to re-synthesise 34 ATP molecules The hydrogen ion (H+) combines with oxygen to form water (H2O) ...
Carbohydrates
... 2. Assists Aeration Sugar denatures egg protein, enabling aeration to occur, e.g. in the making of sponge cakes – the egg when whisked with sugar becomes aerated ...
... 2. Assists Aeration Sugar denatures egg protein, enabling aeration to occur, e.g. in the making of sponge cakes – the egg when whisked with sugar becomes aerated ...
Q1. (a) An enzyme catalyses only one reaction. Explain why
... enzymes. The protein acts as an enzyme by breaking down chitin, a polysaccharide found in the walls of many fungi, to its monomers. Because of the resulting more negative water potential in the cytoplasm of the fungus, this effectively leads to “death by osmosis” of any fungus attacking the grain. ...
... enzymes. The protein acts as an enzyme by breaking down chitin, a polysaccharide found in the walls of many fungi, to its monomers. Because of the resulting more negative water potential in the cytoplasm of the fungus, this effectively leads to “death by osmosis” of any fungus attacking the grain. ...
Biomolecules
... Proteolysis, amino acid pool, metabolic flow of amino acid nitrogen, fate of carbon skeletons, biosynthesis of other amino acid-derived compounds, heme metabolism. Nucleotide metabolism Synthesis of purine and pymiridine nucleotides Degradation of purines and pyrimidines, inhibition of purine and py ...
... Proteolysis, amino acid pool, metabolic flow of amino acid nitrogen, fate of carbon skeletons, biosynthesis of other amino acid-derived compounds, heme metabolism. Nucleotide metabolism Synthesis of purine and pymiridine nucleotides Degradation of purines and pyrimidines, inhibition of purine and py ...
acetyl CoA carboxylase
... Sources of NADPH for Fatty Acid Synthesis 1. One molecule of NADPH is generated for each molecule of acetyl CoA that is transferred from mitochondria to the cytosol (malic enzyme). ...
... Sources of NADPH for Fatty Acid Synthesis 1. One molecule of NADPH is generated for each molecule of acetyl CoA that is transferred from mitochondria to the cytosol (malic enzyme). ...
AMINO ACIDS METABOLISM ** Dr. Mohammed Abdullateef **
... The toxicity is due to the reason that increased concentration of ammonia in the blood and other biological fluids → ammonia difuses into cells, across blood/brain barrier → increased synthesis of glutamate from a-ketoglutarate by glutamate dehydrogenase, increased synthesis of glutamine. Alpha keto ...
... The toxicity is due to the reason that increased concentration of ammonia in the blood and other biological fluids → ammonia difuses into cells, across blood/brain barrier → increased synthesis of glutamate from a-ketoglutarate by glutamate dehydrogenase, increased synthesis of glutamine. Alpha keto ...
Textbook of Biochemistry - OSU Biochemistry and Molecular Biology
... 7.2.1 Glycolysis occurs in all human cells. 7.2.1.1 The overall reaction gl;ucose ––> 2 pyruvate ––>2 actyel CoA 7.2.1.2 glucose + 6O2 + 38 ADP3- + 38 Pi2- ––> 6CO2 + 6 H2O + 38 ATP47.2.1.3 Glucose is metabolized differently in various cells. 7.3 The Glycolytic Pathway 7.3.1 See Fig. 7.6 7.3.2 Glyco ...
... 7.2.1 Glycolysis occurs in all human cells. 7.2.1.1 The overall reaction gl;ucose ––> 2 pyruvate ––>2 actyel CoA 7.2.1.2 glucose + 6O2 + 38 ADP3- + 38 Pi2- ––> 6CO2 + 6 H2O + 38 ATP47.2.1.3 Glucose is metabolized differently in various cells. 7.3 The Glycolytic Pathway 7.3.1 See Fig. 7.6 7.3.2 Glyco ...
Amino Acid Catabolism
... • Leucine is degraded to acetyl CoA and acetoacetate by a pathway whose first two seps are identical to those of valine degradation (Figure 18-11). The third step is the same as the first step of fatty acid oxidation. The fourth step involves an ATPdependent carboxylation, the fifth step is a hydrat ...
... • Leucine is degraded to acetyl CoA and acetoacetate by a pathway whose first two seps are identical to those of valine degradation (Figure 18-11). The third step is the same as the first step of fatty acid oxidation. The fourth step involves an ATPdependent carboxylation, the fifth step is a hydrat ...
Bio Exam 4 Study Guide- Question Format Fatty acid Synthesis
... a. Dephosphorylating acetyl CoA carboxylase; dephosphorylating lipase, preventing TG hydrolysis 43. What other two forms of regulation does FA synthesis have? a. Feed forward and feedback inhibition Synthesis of TG and Membrane Lipids 1. Synthesis of phosphatidic acid starts synthesizing glycerol-3- ...
... a. Dephosphorylating acetyl CoA carboxylase; dephosphorylating lipase, preventing TG hydrolysis 43. What other two forms of regulation does FA synthesis have? a. Feed forward and feedback inhibition Synthesis of TG and Membrane Lipids 1. Synthesis of phosphatidic acid starts synthesizing glycerol-3- ...
Student notes in ppt
... The regulatory protein AMPK is activated by low energy charge in the cell (high levels of AMP). The activity of AMPK is regulated by both AMP binding and by phosphorylation at a highly conserved threonine residue. The enzyme that phosphorylates AMP kinase is functionally referred to as AMP kinase ki ...
... The regulatory protein AMPK is activated by low energy charge in the cell (high levels of AMP). The activity of AMPK is regulated by both AMP binding and by phosphorylation at a highly conserved threonine residue. The enzyme that phosphorylates AMP kinase is functionally referred to as AMP kinase ki ...
New York: Holt, Rinehart and Winston, Inc., 1992.
... formed in matrix from: (1) Oxidative decarboxilation of pyruvate to acetyl CoA (2) Aerobic oxidation of acetyl CoA by the citric acid cycle (3) Oxidation of fatty acids and amino acids ...
... formed in matrix from: (1) Oxidative decarboxilation of pyruvate to acetyl CoA (2) Aerobic oxidation of acetyl CoA by the citric acid cycle (3) Oxidation of fatty acids and amino acids ...
Respiration Lab. eScience Lab 8. Experiments 1 and 2. Tips
... Positive control (glucose): The tube with glucose is the positive control because we already know that yeast can use it in fermentation, so we know that gas should be produced. Therefore, a lot of carbon dioxide gas is produced in the glucose tube. Negative control (water): The tube with water is t ...
... Positive control (glucose): The tube with glucose is the positive control because we already know that yeast can use it in fermentation, so we know that gas should be produced. Therefore, a lot of carbon dioxide gas is produced in the glucose tube. Negative control (water): The tube with water is t ...
Principles of BIOCHEMISTRY
... formed in matrix from: (1) Oxidative decarboxilation of pyruvate to acetyl CoA (2) Aerobic oxidation of acetyl CoA by the citric acid cycle (3) Oxidation of fatty acids and amino acids ...
... formed in matrix from: (1) Oxidative decarboxilation of pyruvate to acetyl CoA (2) Aerobic oxidation of acetyl CoA by the citric acid cycle (3) Oxidation of fatty acids and amino acids ...
Oxidative Phosphorylation in Homogenates of
... Downloaded from cancerres.aacrjournals.org on June 18, 2017. © 1951 American Association for Cancer Research. ...
... Downloaded from cancerres.aacrjournals.org on June 18, 2017. © 1951 American Association for Cancer Research. ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑