Chapter 2b Packet answers
... 7. A chemical reaction can be sped up by adding a substance called a(n) __enzyme/catalyst_, which lowers the amount of activation energy required to start the reaction. 8. The portion of an enzyme molecule into which a specific substrate can fit is called the _active site__. 9. Trypsin is a(n) __enz ...
... 7. A chemical reaction can be sped up by adding a substance called a(n) __enzyme/catalyst_, which lowers the amount of activation energy required to start the reaction. 8. The portion of an enzyme molecule into which a specific substrate can fit is called the _active site__. 9. Trypsin is a(n) __enz ...
Ch 8 Slides - people.iup.edu
... • To do work, cells manage energy resources by energy coupling, the use of an exergonic process to drive an endergonic one • Most energy coupling in cells is mediated by ATPbecause ATP hydrolysis is highly exergonic ...
... • To do work, cells manage energy resources by energy coupling, the use of an exergonic process to drive an endergonic one • Most energy coupling in cells is mediated by ATPbecause ATP hydrolysis is highly exergonic ...
reaction
... production of glycolysis. (Since the sugar splitting in step 4, all products are doubled. Therefore, this step actually repays the earlier investment of two ATP molecules.) ...
... production of glycolysis. (Since the sugar splitting in step 4, all products are doubled. Therefore, this step actually repays the earlier investment of two ATP molecules.) ...
Ch 8 Slides
... • To do work, cells manage energy resources by energy coupling, the use of an exergonic process to drive an endergonic one • Most energy coupling in cells is mediated by ATPbecause ATP hydrolysis is highly exergonic ...
... • To do work, cells manage energy resources by energy coupling, the use of an exergonic process to drive an endergonic one • Most energy coupling in cells is mediated by ATPbecause ATP hydrolysis is highly exergonic ...
Additional Science Biology Summary
... Used in respiration to release energy, used to make starch an insoluble storage sugar, used to make cellulose for the cell wall, combined with nitrates to make amino acids for proteins, used to make fats and oils. The three limiting factors are………….. Carbon dioxide concentration, light intensity and ...
... Used in respiration to release energy, used to make starch an insoluble storage sugar, used to make cellulose for the cell wall, combined with nitrates to make amino acids for proteins, used to make fats and oils. The three limiting factors are………….. Carbon dioxide concentration, light intensity and ...
Energy and Muscle Contraction
... Therefore, during this time, cellular respiration will be going at capacity, limited only by oxygen restraints. Glycolysis, on the other hand, will proceed at an accelerated rate for the purpose of gaining extra ATP. Note that during this time, pyruvate will be fed into the mitochondria as fast as s ...
... Therefore, during this time, cellular respiration will be going at capacity, limited only by oxygen restraints. Glycolysis, on the other hand, will proceed at an accelerated rate for the purpose of gaining extra ATP. Note that during this time, pyruvate will be fed into the mitochondria as fast as s ...
anaerobic respiration
... Two CO2 molecules are fixed and converted into one acetyl-CoA via the hydroxypropionate pathway. . The net result is that three CO2 molecules are converted into one pyruvic acid molecule. ...
... Two CO2 molecules are fixed and converted into one acetyl-CoA via the hydroxypropionate pathway. . The net result is that three CO2 molecules are converted into one pyruvic acid molecule. ...
From Fig - Jiamusi University
... the inside to the outside of the inner mitochondrial membrane. The electrochemical potential difference resulting from the asymmetric distribution of the hydrogen ions is used to drive the mechanism responsible for the formation of ATP (Fig. 7-10). From Fig. 7-10 the oxidation in the respiratory cha ...
... the inside to the outside of the inner mitochondrial membrane. The electrochemical potential difference resulting from the asymmetric distribution of the hydrogen ions is used to drive the mechanism responsible for the formation of ATP (Fig. 7-10). From Fig. 7-10 the oxidation in the respiratory cha ...
Integrating the universal metabolism into a phylogenetic analysis
... The darwinian concept of ‘‘descent with modification’’ applies to metabolic pathways: pathways sharing similarities must have inherited them from an exclusive, hypothetical ancestral pathway. Comparative anatomy of biochemical pathways is performed using five criteria of homology. Primary homologies ...
... The darwinian concept of ‘‘descent with modification’’ applies to metabolic pathways: pathways sharing similarities must have inherited them from an exclusive, hypothetical ancestral pathway. Comparative anatomy of biochemical pathways is performed using five criteria of homology. Primary homologies ...
Glucose metabolism in Trypanosoma cruzi
... somal isoforms of PGK in T. cruzi; however, they would not be enough to keep the ATP concentration balanced to ensure that glycolysis would proceed. PEP (phosphoenolpyruvate), obtained from 3‑phosphoglycerate in the cytosol, can produce one ATP molecule in the PK reaction. However, it is generally a ...
... somal isoforms of PGK in T. cruzi; however, they would not be enough to keep the ATP concentration balanced to ensure that glycolysis would proceed. PEP (phosphoenolpyruvate), obtained from 3‑phosphoglycerate in the cytosol, can produce one ATP molecule in the PK reaction. However, it is generally a ...
What Are the Health Benefits of Physical Activity?
... Guidelines & Goal Setting Activities for Achieving FITT Principle ...
... Guidelines & Goal Setting Activities for Achieving FITT Principle ...
PROTEIN TURNOVER AND NITROGEN ECONOMY - U
... - proteins metabolism has a balance between body’s energy and synthetic needs - dietary protein required to synthesize endogenous proteins (albumin, myosin, actin) - essential amino acids cannot be synthesize by body; others can be synthesized from carbon sources -table - protein balance relations ...
... - proteins metabolism has a balance between body’s energy and synthetic needs - dietary protein required to synthesize endogenous proteins (albumin, myosin, actin) - essential amino acids cannot be synthesize by body; others can be synthesized from carbon sources -table - protein balance relations ...
CHAPTER - 6 LIFE PROCESSES
... The energy released during respiration is used to make ATP molecules (Adenosine tri phosphate) from ADP molecules (Adenosine di phosphate) and inorganic phosphate. Energy ADP + Phosphate ...
... The energy released during respiration is used to make ATP molecules (Adenosine tri phosphate) from ADP molecules (Adenosine di phosphate) and inorganic phosphate. Energy ADP + Phosphate ...
PPT
... • The chain functions as a chemical machine that uses energy released by the “fall” of electrons to pump hydrogen ions across the inner mitochondrial ...
... • The chain functions as a chemical machine that uses energy released by the “fall” of electrons to pump hydrogen ions across the inner mitochondrial ...
Pathways of Glucose Assimilation in Puccinia graminis
... evaporated to dryness in a scintillation vial, 10 ml scintillant solution was added and the radioactivity was estimated. The aqueous fraction was separated into neutral, anionic and cationic fractions by ion exchange chromatography as described by Neal & Beevers (1961). The fractions were evaporated ...
... evaporated to dryness in a scintillation vial, 10 ml scintillant solution was added and the radioactivity was estimated. The aqueous fraction was separated into neutral, anionic and cationic fractions by ion exchange chromatography as described by Neal & Beevers (1961). The fractions were evaporated ...
hanan abas
... Urine ;Its’ complex aqueous solution of organic and inorganic substances resulting from the metabolism processes in the Body . General properties of urine; The specific Gravity of the urine varies between(1.003_1.030) and pH ranges from (4.6_8) with an average value (6.3). Urine is normally pale yel ...
... Urine ;Its’ complex aqueous solution of organic and inorganic substances resulting from the metabolism processes in the Body . General properties of urine; The specific Gravity of the urine varies between(1.003_1.030) and pH ranges from (4.6_8) with an average value (6.3). Urine is normally pale yel ...
Spotlight on metabolic remodelling in heart failure
... out that, based on transgenic models and pharmacological studies, evidence is available that increasing glucose oxidation under conditions of hypertrophy is not harmful, but the therapeutic potential of metabolic strategies lies more in the arena of general improvement in oxidative capacity. This co ...
... out that, based on transgenic models and pharmacological studies, evidence is available that increasing glucose oxidation under conditions of hypertrophy is not harmful, but the therapeutic potential of metabolic strategies lies more in the arena of general improvement in oxidative capacity. This co ...
Biochemistry Study Guide NITROGEN METABOLISM
... 2 ATP are required. Basically these are used to "charge" or "activate" ammonia with a highenergy phosphate bond, before we subsequently start urea synthesis. N-Acetylglutamate is absolutely required as a cofactor. This compound also serves a regulatory role in urea synthesis. The rate of carba ...
... 2 ATP are required. Basically these are used to "charge" or "activate" ammonia with a highenergy phosphate bond, before we subsequently start urea synthesis. N-Acetylglutamate is absolutely required as a cofactor. This compound also serves a regulatory role in urea synthesis. The rate of carba ...
ATP Synthesis
... (strictly, HPO42-) via “oxidative phosphorylation” - Coupling the free energy stored in the proton gradient to ATP synthesis is carried out by an enzyme called “ATP synthase”—which can be essentially viewed as Complex V located downstream of Complexes I-IV in the ETC - Embedded within the IMM and pr ...
... (strictly, HPO42-) via “oxidative phosphorylation” - Coupling the free energy stored in the proton gradient to ATP synthesis is carried out by an enzyme called “ATP synthase”—which can be essentially viewed as Complex V located downstream of Complexes I-IV in the ETC - Embedded within the IMM and pr ...
Nutrients
... compounds that make up foods and which are essential for life. • Nutrients include: • Carbohydrates • Lipids • Proteins • Vitamins • Minerals ...
... compounds that make up foods and which are essential for life. • Nutrients include: • Carbohydrates • Lipids • Proteins • Vitamins • Minerals ...
8.1 Energy and Life
... Chemical Energy and ATP Energy is the ability to do work. Organisms need energy to stay alive. Adenosine triphosphate (ATP) is a chemical compound cells use to store and release energy. • An ATP molecule consists of adenine, the sugar ribose, and three phosphate groups. • Cells store energy by addin ...
... Chemical Energy and ATP Energy is the ability to do work. Organisms need energy to stay alive. Adenosine triphosphate (ATP) is a chemical compound cells use to store and release energy. • An ATP molecule consists of adenine, the sugar ribose, and three phosphate groups. • Cells store energy by addin ...
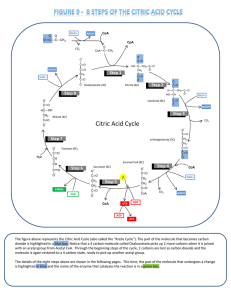
Adenosine triphosphate Adenosine triphosphate Adenosine
... results in the synthesis of between 2-3 ATP molecules, and the oxidation of one FADH2 yields between 1-2 ATP molecules.[22] The majority of cellular ATP is generated by this process. Although the citric acid cycle itself does not involve molecular oxygen, it is an obligately aerobic process because ...
... results in the synthesis of between 2-3 ATP molecules, and the oxidation of one FADH2 yields between 1-2 ATP molecules.[22] The majority of cellular ATP is generated by this process. Although the citric acid cycle itself does not involve molecular oxygen, it is an obligately aerobic process because ...
Glycolysis
Glycolysis (from glycose, an older term for glucose + -lysis degradation) is the metabolic pathway that converts glucose C6H12O6, into pyruvate, CH3COCOO− + H+. The free energy released in this process is used to form the high-energy compounds ATP (adenosine triphosphate) and NADH (reduced nicotinamide adenine dinucleotide).Glycolysis is a determined sequence of ten enzyme-catalyzed reactions. The intermediates provide entry points to glycolysis. For example, most monosaccharides, such as fructose and galactose, can be converted to one of these intermediates. The intermediates may also be directly useful. For example, the intermediate dihydroxyacetone phosphate (DHAP) is a source of the glycerol that combines with fatty acids to form fat.Glycolysis is an oxygen independent metabolic pathway, meaning that it does not use molecular oxygen (i.e. atmospheric oxygen) for any of its reactions. However the products of glycolysis (pyruvate and NADH + H+) are sometimes disposed of using atmospheric oxygen. When molecular oxygen is used in the disposal of the products of glycolysis the process is usually referred to as aerobic, whereas if the disposal uses no oxygen the process is said to be anaerobic. Thus, glycolysis occurs, with variations, in nearly all organisms, both aerobic and anaerobic. The wide occurrence of glycolysis indicates that it is one of the most ancient metabolic pathways. Indeed, the reactions that constitute glycolysis and its parallel pathway, the pentose phosphate pathway, occur metal-catalyzed under the oxygen-free conditions of the Archean oceans, also in the absence of enzymes. Glycolysis could thus have originated from chemical constraints of the prebiotic world.Glycolysis occurs in most organisms in the cytosol of the cell. The most common type of glycolysis is the Embden–Meyerhof–Parnas (EMP pathway), which was discovered by Gustav Embden, Otto Meyerhof, and Jakub Karol Parnas. Glycolysis also refers to other pathways, such as the Entner–Doudoroff pathway and various heterofermentative and homofermentative pathways. However, the discussion here will be limited to the Embden–Meyerhof–Parnas pathway.The entire glycolysis pathway can be separated into two phases: The Preparatory Phase – in which ATP is consumed and is hence also known as the investment phase The Pay Off Phase – in which ATP is produced.↑ ↑ 2.0 2.1 ↑ ↑ ↑ ↑ ↑ ↑























