Amino Acids 14.5 * 14.8
... Produces an Amide. The two Amino Acids are joined together by an peptide bond. (the linking of two amino acids) Produces dipeptide. ...
... Produces an Amide. The two Amino Acids are joined together by an peptide bond. (the linking of two amino acids) Produces dipeptide. ...
Endoproteinase pro-C-catalyzed peptide bond
... enzymes, protease-catalyzed peptide bond formation has proved to be an attractive alternative to chemical methods.1,2 Proteases catalyze peptide synthesis under mild reaction conditions and without time-consuming side chain protection strategy. Proline is an essential part of many biologically activ ...
... enzymes, protease-catalyzed peptide bond formation has proved to be an attractive alternative to chemical methods.1,2 Proteases catalyze peptide synthesis under mild reaction conditions and without time-consuming side chain protection strategy. Proline is an essential part of many biologically activ ...
Peptides and Protein Primary Structure
... • Adjacent R-groups interact with each other. • This interaction leads to the discrete f,y angles about each alpha carbon. • The collective effect of these f,y angles gives rise to secondary structure:α-helix and β-conformation ...
... • Adjacent R-groups interact with each other. • This interaction leads to the discrete f,y angles about each alpha carbon. • The collective effect of these f,y angles gives rise to secondary structure:α-helix and β-conformation ...
Peptides and Protein Primary Structure
... • Adjacent R-groups interact with each other. • This interaction leads to the discrete φ,ψ ψ angles about each alpha carbon. • The collective effect of these φ,ψ angles gives rise to secondary structure:α-helix and β-conformation ...
... • Adjacent R-groups interact with each other. • This interaction leads to the discrete φ,ψ ψ angles about each alpha carbon. • The collective effect of these φ,ψ angles gives rise to secondary structure:α-helix and β-conformation ...
No Slide Title
... The amino acid sequence (also called primary structure) of a protein is the order of the amino acids in the protein chain. The sequence is always read from the N-terminus to the Cterminus of the protein. ...
... The amino acid sequence (also called primary structure) of a protein is the order of the amino acids in the protein chain. The sequence is always read from the N-terminus to the Cterminus of the protein. ...
Biology - PHA Science
... get grades for the best 4 (but you must do #1). 1. Proteins are essential for all life on earth. a) Diagram an amino acid and label its functional groups. What is the importance of the R group? b) Describe the four different levels of protein structure. What kind of chemical interactions (e.g. hydro ...
... get grades for the best 4 (but you must do #1). 1. Proteins are essential for all life on earth. a) Diagram an amino acid and label its functional groups. What is the importance of the R group? b) Describe the four different levels of protein structure. What kind of chemical interactions (e.g. hydro ...
lec---10
... • Protein is a polymer of amino acids (constructed from 20 amino acids) (to form Polypeptides). • These components include a hydrogen atom, a carboxyl group, an amino group, and a variable R group (or side chain). General Formula of the ...
... • Protein is a polymer of amino acids (constructed from 20 amino acids) (to form Polypeptides). • These components include a hydrogen atom, a carboxyl group, an amino group, and a variable R group (or side chain). General Formula of the ...
lipid3 - ChemEd DL
... A water molecule is eliminated, leaving a bond between the acid carbon of the first amino acid and the amine nitrogen of the second. The peptide bond is left between the two amino acids. ...
... A water molecule is eliminated, leaving a bond between the acid carbon of the first amino acid and the amine nitrogen of the second. The peptide bond is left between the two amino acids. ...
Reading Guide
... 27. Aromatic amino acids are both keto- and glucogenic because they are broken down into ___________________ and either ______________ or _______________. 28. Why is excess nitrogen from metabolic processes not simply excreted as ammonia? 29. What is glutamate’s particular role in nitrogen eliminat ...
... 27. Aromatic amino acids are both keto- and glucogenic because they are broken down into ___________________ and either ______________ or _______________. 28. Why is excess nitrogen from metabolic processes not simply excreted as ammonia? 29. What is glutamate’s particular role in nitrogen eliminat ...
PowerPoint bemutató
... Isomerase (PDI) • Provides mechanism for breaking incorrectly paired disulfide bonds. ...
... Isomerase (PDI) • Provides mechanism for breaking incorrectly paired disulfide bonds. ...
PowerPoint bemutató
... Isomerase (PDI) • Provides mechanism for breaking incorrectly paired disulfide bonds. ...
... Isomerase (PDI) • Provides mechanism for breaking incorrectly paired disulfide bonds. ...
462a Reading and Homework Assignment 3
... Most of the residues with “unfavorable” , angles probably involve glycine residues. c. Which of the two Ramachandran diagrams corresponds to which of the two proteins shown below? Explain. Protein 1 (Ramachandran plot A) is T4 lysozyme, which is almost all -helical. Protein 2 (Ramachandran plot ...
... Most of the residues with “unfavorable” , angles probably involve glycine residues. c. Which of the two Ramachandran diagrams corresponds to which of the two proteins shown below? Explain. Protein 1 (Ramachandran plot A) is T4 lysozyme, which is almost all -helical. Protein 2 (Ramachandran plot ...
Amino Acids, Peptides and Proteins
... 1. You have a solution of tyrosine. You decided to modify Y by methylation of the carboxyl, explain how this would change the acid-base titration of this molecule. 2. You have a solution of tyrosine. You decided to modify Y by methylation of the “alcohol”, explain how this would change the acid-base ...
... 1. You have a solution of tyrosine. You decided to modify Y by methylation of the carboxyl, explain how this would change the acid-base titration of this molecule. 2. You have a solution of tyrosine. You decided to modify Y by methylation of the “alcohol”, explain how this would change the acid-base ...
CHEM501- Introduction to Biochemistry – Exam 1 w
... A) Cystine forms when the —CH2—SH R group is oxidized to form a —CH2—S—S— CH2— disulfide bridge between two cysteines. B) Cystine is an example of a nonstandard amino acid, derived by linking two standard amino acids. C) Cystine is formed by the oxidation of the carboxylic acid group on cysteine. D) ...
... A) Cystine forms when the —CH2—SH R group is oxidized to form a —CH2—S—S— CH2— disulfide bridge between two cysteines. B) Cystine is an example of a nonstandard amino acid, derived by linking two standard amino acids. C) Cystine is formed by the oxidation of the carboxylic acid group on cysteine. D) ...
Review Sheet - Phillips Scientific Methods
... o Kinks can occur where double bond is present o Some can be acidic or basic –depending on balance of functional groups o Some hydrophilic, some hydrophobic o A peptide bond is formed during dehydration synthesis Bond between a carbon and a nitrogen from loss of water. Actually more ionic than cov ...
... o Kinks can occur where double bond is present o Some can be acidic or basic –depending on balance of functional groups o Some hydrophilic, some hydrophobic o A peptide bond is formed during dehydration synthesis Bond between a carbon and a nitrogen from loss of water. Actually more ionic than cov ...
Remediation/Corrections Packet
... Lipids are large, nonpolar (won't dissolve in water) molecules. Phospholipids make up cell membranes. Lipids also serve as waxy coverings and steroids. Lipids have more carbon and hydrogen atoms than oxygen atoms. Fats are made of a glycerol (alcohol) and three fatty acid chains. This subunit is ca ...
... Lipids are large, nonpolar (won't dissolve in water) molecules. Phospholipids make up cell membranes. Lipids also serve as waxy coverings and steroids. Lipids have more carbon and hydrogen atoms than oxygen atoms. Fats are made of a glycerol (alcohol) and three fatty acid chains. This subunit is ca ...
Macromolecules practice worksheet key
... Saturated fats have the maximum amount of hydrogens bound to the carbons of the F.A. chains, therefore, they lack double bonds and can pack tightly forming solids at room temp. and unsaturated fats have less hydrogens bound to the carbons of F.A. chains, therefore, they do have double bonds and do n ...
... Saturated fats have the maximum amount of hydrogens bound to the carbons of the F.A. chains, therefore, they lack double bonds and can pack tightly forming solids at room temp. and unsaturated fats have less hydrogens bound to the carbons of F.A. chains, therefore, they do have double bonds and do n ...
short chain polypeptide test
... North Cottage 11 Dovers Green Road Reigate Surrey RH2 8BU Tel: 01737 226338 ...
... North Cottage 11 Dovers Green Road Reigate Surrey RH2 8BU Tel: 01737 226338 ...
PP-Protein Synthesis
... Proteins have MANY different functions Enzymes to help control/speed up chemical reactions Help to build and repair cell structures Determine the structure & function of living organisms ...
... Proteins have MANY different functions Enzymes to help control/speed up chemical reactions Help to build and repair cell structures Determine the structure & function of living organisms ...
Ch 30 reading guide
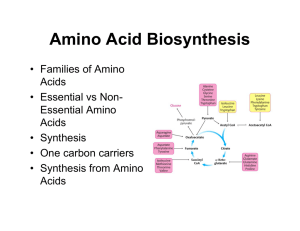
... in which branched amino acid nitrogen is incorporated into the amino acid ___________________, then to the amino acid _____________________ for transport through the blood to the liver. 6. Another carrier of nitrogen is the amino acid ________________________, which is made when ammonia is incorpora ...
... in which branched amino acid nitrogen is incorporated into the amino acid ___________________, then to the amino acid _____________________ for transport through the blood to the liver. 6. Another carrier of nitrogen is the amino acid ________________________, which is made when ammonia is incorpora ...