02 Chemistry b - Crestwood Local Schools
... Lipoproteins – transport fatty acids and cholesterol in ...
... Lipoproteins – transport fatty acids and cholesterol in ...
LOYOLA COLLEGE (AUTONOMOUS), CHENNAI – 600 034
... 26. Classify amino acids and lipids based on the structure with suitable examples. 27. Write in detail about the steps involved in complete oxidation of glucose and regulation. 28. Describe the principle and instrumentation of HPLC and GLC. 29. Elaborate on 2D gel electrophoresis of proteins. ...
... 26. Classify amino acids and lipids based on the structure with suitable examples. 27. Write in detail about the steps involved in complete oxidation of glucose and regulation. 28. Describe the principle and instrumentation of HPLC and GLC. 29. Elaborate on 2D gel electrophoresis of proteins. ...
The Human Genome Project
... outcomes: – 21,000 genes in the human genome – Only about 2% of these code for proteins – One gene is able to code for many proteins – About 8 million single nucleotide polymorphisms (SNPs) ...
... outcomes: – 21,000 genes in the human genome – Only about 2% of these code for proteins – One gene is able to code for many proteins – About 8 million single nucleotide polymorphisms (SNPs) ...
37151
... The term ‘Proteome’ coined in 1994 Complete set of proteins exported and modified following expression, by entire genome in lifetime of a cell Proteomics is usually carried out to study the complement of protein expressed by a cell at any one time or at a particular stage ...
... The term ‘Proteome’ coined in 1994 Complete set of proteins exported and modified following expression, by entire genome in lifetime of a cell Proteomics is usually carried out to study the complement of protein expressed by a cell at any one time or at a particular stage ...
4NucleicAcidsProteins - San Elijo Elementary School
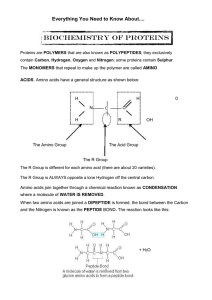
... • Structure and function determined by # and sequence of AA 1. Primary structure (AA sequence) 2. Secondary structure (Hydrogen to Oxygen) • Primary polypeptide coil and fold • Due to hydrogen bonds between adjacent peptide bonds O H (NOT r-side chains) • Special: alpha-helix, beta-pleated ...
... • Structure and function determined by # and sequence of AA 1. Primary structure (AA sequence) 2. Secondary structure (Hydrogen to Oxygen) • Primary polypeptide coil and fold • Due to hydrogen bonds between adjacent peptide bonds O H (NOT r-side chains) • Special: alpha-helix, beta-pleated ...
AS Biology - Everything Protein
... PRIMARY STRUCTURE is the AMINO ACID SEQUENCE; peptide bonds are present in this level of structure. SECONDARY STRUCTURE is how the primary structure folds for the first time. The two most common secondary structure folds are shown below: ...
... PRIMARY STRUCTURE is the AMINO ACID SEQUENCE; peptide bonds are present in this level of structure. SECONDARY STRUCTURE is how the primary structure folds for the first time. The two most common secondary structure folds are shown below: ...
Proteins Multiple choice Proteins can be classified as Polyesters
... maltose. The pH of saliva is about 7, which is close to the optimum temperature of an enzyme. Amylase stops functioning when it enters the stomach which has a pH of 2. What happens to the enzyme on entering the stomach which would cause it to ...
... maltose. The pH of saliva is about 7, which is close to the optimum temperature of an enzyme. Amylase stops functioning when it enters the stomach which has a pH of 2. What happens to the enzyme on entering the stomach which would cause it to ...
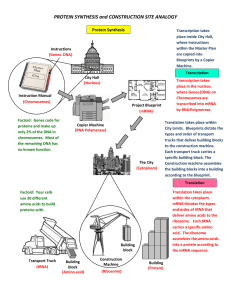
PROTEIN SYNTHESIS and CONSTRUCTION SITE ANALOGY
... within the Master Plan are copied into Blueprints by a Copier Machine. ...
... within the Master Plan are copied into Blueprints by a Copier Machine. ...
Biology Section 2 Molecules of Life Carbohydrates Carbohydrates
... o Polypeptides- long string of amino acids o Protein shape influenced by bonding, solvent, temperature Enzymes o Enzymes- RNA or protein catalysts o Physical fit between enzyme and substrate (substance being catalyzed) o Active site- folds o Slight change in shape weakens chemical bonds o Enzymes ...
... o Polypeptides- long string of amino acids o Protein shape influenced by bonding, solvent, temperature Enzymes o Enzymes- RNA or protein catalysts o Physical fit between enzyme and substrate (substance being catalyzed) o Active site- folds o Slight change in shape weakens chemical bonds o Enzymes ...
MTC25 - Intracellular Processing
... Newly formed polypeptides / proteins are released from ribosomal sites in the nucleus to be transported to other areas of the cell Translocation to the rough endoplasmic reticulum (ER) occurs during protein synthesis: o A signal sequence is formed on the nascent protein which is identified and bound ...
... Newly formed polypeptides / proteins are released from ribosomal sites in the nucleus to be transported to other areas of the cell Translocation to the rough endoplasmic reticulum (ER) occurs during protein synthesis: o A signal sequence is formed on the nascent protein which is identified and bound ...
organic compounds outline
... used in protein function of individual proteins _____________________ – copying the DNA gene to a strand of mRNA ____________________ – ribosomes assemble amino acids into the correct sequence Knows the sequence by the mRNA code Problems – __________________ Def: changes in the DNA seque ...
... used in protein function of individual proteins _____________________ – copying the DNA gene to a strand of mRNA ____________________ – ribosomes assemble amino acids into the correct sequence Knows the sequence by the mRNA code Problems – __________________ Def: changes in the DNA seque ...
Molecules and Life Quiz 3C
... Proteins They are building blocks of many structures in organisms. Your muscles contain large amounts of protein. ...
... Proteins They are building blocks of many structures in organisms. Your muscles contain large amounts of protein. ...
Nonstandard amino acids are found in modified proteins
... points per amino acid 2. Add in nitrogen: 1st point and every 3rd following 3. Add hydrogens and oxygens to complete the backbone 4. Add side chains: draw “up and out, down and back” for L-amino acids (opposite for D) ...
... points per amino acid 2. Add in nitrogen: 1st point and every 3rd following 3. Add hydrogens and oxygens to complete the backbone 4. Add side chains: draw “up and out, down and back” for L-amino acids (opposite for D) ...
Amino acids have many roles in living organisms
... The properties of the amino acids determine which can interact and how. The connectivity (sequence) limits the possible interactions and directs the position of the polypeptide chain. ...
... The properties of the amino acids determine which can interact and how. The connectivity (sequence) limits the possible interactions and directs the position of the polypeptide chain. ...
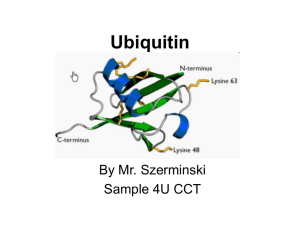
Ubiquitin
... Topics to be discussed • General info: - it is a regulatory protein that has been found in almost all tissues of eukaryotes - one of its functions: it directs protein recycling - can attach to proteins and label them for destruction. - discovery won the Nobel Prize for chemistry in 2004 ...
... Topics to be discussed • General info: - it is a regulatory protein that has been found in almost all tissues of eukaryotes - one of its functions: it directs protein recycling - can attach to proteins and label them for destruction. - discovery won the Nobel Prize for chemistry in 2004 ...
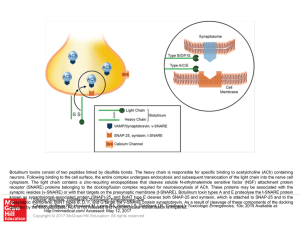
Slide () - AccessEmergency Medicine
... Botulinum toxins consist of two peptides linked by disulfide bonds. The heavy chain is responsible for specific binding to acetylcholine (ACh) containing neurons. Following binding to the cell surface, the entire complex undergoes endocytosis and subsequent translocation of the light chain into the ...
... Botulinum toxins consist of two peptides linked by disulfide bonds. The heavy chain is responsible for specific binding to acetylcholine (ACh) containing neurons. Following binding to the cell surface, the entire complex undergoes endocytosis and subsequent translocation of the light chain into the ...
Unit 3 Biology - moleculesoflife2
... dimensional structures. The shape into which a protein naturally folds is known as its ……………………….., which is determined by its sequence of amino acids. Thus proteins are known as …………………………, with amino acids being the monomers. Biochemists refer to four distinct aspects of protein structure. 1. Prim ...
... dimensional structures. The shape into which a protein naturally folds is known as its ……………………….., which is determined by its sequence of amino acids. Thus proteins are known as …………………………, with amino acids being the monomers. Biochemists refer to four distinct aspects of protein structure. 1. Prim ...
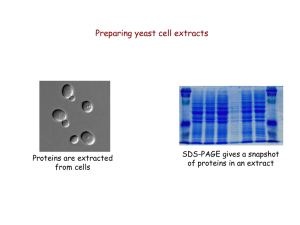
Determination of Proteins
... OBJECTIVES • Master how to determine the conc. of protein in serum/plasma. • Important of protein estimation in health and various diseases. • Understand the principles behind protein estimation. ...
... OBJECTIVES • Master how to determine the conc. of protein in serum/plasma. • Important of protein estimation in health and various diseases. • Understand the principles behind protein estimation. ...
Proteins
... Factors that must be supplied in the diet for the body to be able to synthesis PROTEİN include : 1 . all E.a.a consume simultaneously and in proper amount 2 . an adequate total amount of protein to supply amine groups to synthesis non – E.a.a 3 . adequate of CHO & FAT to spare protein being used to ...
... Factors that must be supplied in the diet for the body to be able to synthesis PROTEİN include : 1 . all E.a.a consume simultaneously and in proper amount 2 . an adequate total amount of protein to supply amine groups to synthesis non – E.a.a 3 . adequate of CHO & FAT to spare protein being used to ...
Biomolecules Worksheet
... 3). There are 3 main groups of amino acids in cells: non-polar, polar, charged. Explain what you would look for in an amino acid to identify it as one of these types. ...
... 3). There are 3 main groups of amino acids in cells: non-polar, polar, charged. Explain what you would look for in an amino acid to identify it as one of these types. ...
John Torri Basic Nutrition Special Topic: Protein November 13 2014
... every day. Amino acids are the building blocks for protein. There are two types of amino acids, essential, and non-essential. Essential amino acids are those that are “essential” in the diet. In other words, our bodies cannot create them through our own metabolism. The main essential amino acids are ...
... every day. Amino acids are the building blocks for protein. There are two types of amino acids, essential, and non-essential. Essential amino acids are those that are “essential” in the diet. In other words, our bodies cannot create them through our own metabolism. The main essential amino acids are ...
L2_Principle of protein folding in the cellular environment
... • Proteins that help the folding of other proteins, usually through cycles of binding and release, without forming part of their final native structure. • Increase in the efficiency, not the specificity, of protein folding • Change in emphasis from post-translational modification to co-translational ...
... • Proteins that help the folding of other proteins, usually through cycles of binding and release, without forming part of their final native structure. • Increase in the efficiency, not the specificity, of protein folding • Change in emphasis from post-translational modification to co-translational ...
The cost of life is energy.
... price” of living by CATALYZING (or helping) reactions they need to stay alive. These reactions are called the METABOLISM. • Enzymes work to SYNTHESIZE molecules and break them apart. ...
... price” of living by CATALYZING (or helping) reactions they need to stay alive. These reactions are called the METABOLISM. • Enzymes work to SYNTHESIZE molecules and break them apart. ...
Protein

Proteins (/ˈproʊˌtiːnz/ or /ˈproʊti.ɨnz/) are large biomolecules, or macromolecules, consisting of one or more long chains of amino acid residues. Proteins perform a vast array of functions within living organisms, including catalyzing metabolic reactions, DNA replication, responding to stimuli, and transporting molecules from one location to another. Proteins differ from one another primarily in their sequence of amino acids, which is dictated by the nucleotide sequence of their genes, and which usually results in protein folding into a specific three-dimensional structure that determines its activity.A linear chain of amino acid residues is called a polypeptide. A protein contains at least one long polypeptide. Short polypeptides, containing less than about 20-30 residues, are rarely considered to be proteins and are commonly called peptides, or sometimes oligopeptides. The individual amino acid residues are bonded together by peptide bonds and adjacent amino acid residues. The sequence of amino acid residues in a protein is defined by the sequence of a gene, which is encoded in the genetic code. In general, the genetic code specifies 20 standard amino acids; however, in certain organisms the genetic code can include selenocysteine and—in certain archaea—pyrrolysine. Shortly after or even during synthesis, the residues in a protein are often chemically modified by posttranslational modification, which alters the physical and chemical properties, folding, stability, activity, and ultimately, the function of the proteins. Sometimes proteins have non-peptide groups attached, which can be called prosthetic groups or cofactors. Proteins can also work together to achieve a particular function, and they often associate to form stable protein complexes.Once formed, proteins only exist for a certain period of time and are then degraded and recycled by the cell's machinery through the process of protein turnover. A protein's lifespan is measured in terms of its half-life and covers a wide range. They can exist for minutes or years with an average lifespan of 1–2 days in mammalian cells. Abnormal and or misfolded proteins are degraded more rapidly either due to being targeted for destruction or due to being unstable.Like other biological macromolecules such as polysaccharides and nucleic acids, proteins are essential parts of organisms and participate in virtually every process within cells. Many proteins are enzymes that catalyze biochemical reactions and are vital to metabolism. Proteins also have structural or mechanical functions, such as actin and myosin in muscle and the proteins in the cytoskeleton, which form a system of scaffolding that maintains cell shape. Other proteins are important in cell signaling, immune responses, cell adhesion, and the cell cycle. Proteins are also necessary in animals' diets, since animals cannot synthesize all the amino acids they need and must obtain essential amino acids from food. Through the process of digestion, animals break down ingested protein into free amino acids that are then used in metabolism.Proteins may be purified from other cellular components using a variety of techniques such as ultracentrifugation, precipitation, electrophoresis, and chromatography; the advent of genetic engineering has made possible a number of methods to facilitate purification. Methods commonly used to study protein structure and function include immunohistochemistry, site-directed mutagenesis, X-ray crystallography, nuclear magnetic resonance and mass spectrometry.